Developmental and thermal regulation of the maize heat shock protein, HSP101
- PMID: 11706162
- PMCID: PMC129251
Developmental and thermal regulation of the maize heat shock protein, HSP101
Abstract
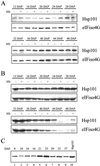
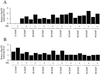
The plant heat stress protein, Hsp101, and the yeast ortholog, Hsp104, are required to confer thermotolerance in plants and yeast (Saccharomyces cerevisiae), respectively. In addition to its function during stress, Hsp101 is developmentally regulated in plants although its function during development is not known. To determine how the expression of Hsp101 is regulated in cereals, we investigated the Hsp101 expression profile in developing maize (Zea mays). Hsp101 protein was most abundant in the developing tassel, ear, silks, endosperm, and embryo. It was less abundant in the vegetative and floral meristematic regions and was present at only a low level in the anthers and tassel at anthesis, mature pollen, roots, and leaves. As expected, heat treatment resulted in an increase in the level of Hsp101 protein in several organs. In expanding foliar leaves, husk leaves, the tassel at the premeiosis stage of development, or pre-anthesis anthers, however, the heat-mediated increase in protein was not accompanied by an equivalent increase in mRNA. In contrast, the level of Hsp101 transcript increased in the tassel at anthesis following a heat stress without an increase in Hsp101 protein. In other organs such as the vegetative and floral meristematic regions, fully expanded foliar leaves, the young ear, and roots, the heat-induced increase in Hsp101 protein was accompanied by a corresponding increase in Hsp101 transcript level. However, anthers at anthesis, mature pollen, developing endosperm, and embryos largely failed to mount a heat stress response at the level of Hsp101 protein or mRNA, indicating that Hsp101 expression is not heat inducible in these organs. In situ RNA localization analysis revealed that Hsp101 mRNA accumulated in the subaleurone and aleurone of developing kernels and was highest in the root cap meristem and quiescent center of heat-stressed roots. These data suggest an organ-specific control of Hsp101 expression during development and following a heat stress through mechanisms that may include posttranscriptional regulation.
Figures

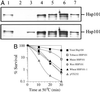


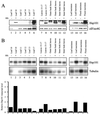
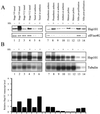
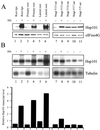


References
-
- Becker J, Craig EA. Heat-shock proteins as molecular chaperones. Eur J Biochem. 1994;219:11–23. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Browning KS, Lax SR, Ravel JM. Identification of two messenger RNA cap binding proteins in wheat germ: evidence that the 28-kDa subunit of eIF-4B and the 26-kDa subunit of eIF-4F are antigenically distinct polypeptides. J Biol Chem. 1987;262:11228–11232. - PubMed
-
- Caliebe A, Soill J. News in chloroplast protein import. Plant Mol Biol. 1999;39:641–645. - PubMed
-
- Caplan AJ. Hsp90's secrets unfold: new insights from structural and functional studies. Trends Cell Biol. 1999;9:262–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
