Environmental regulation of lateral root initiation in Arabidopsis
- PMID: 11706172
- PMCID: PMC129261
Environmental regulation of lateral root initiation in Arabidopsis
Abstract
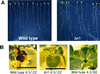
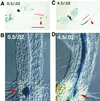
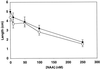
Plant morphology is dramatically influenced by environmental signals. The growth and development of the root system is an excellent example of this developmental plasticity. Both the number and placement of lateral roots are highly responsive to nutritional cues. This indicates that there must be a signal transduction pathway that interprets complex environmental conditions and makes the "decision" to form a lateral root at a particular time and place. Lateral roots originate from differentiated cells in adult tissues. These cells must reenter the cell cycle, proliferate, and redifferentiate to produce all of the cell types that make up a new organ. Almost nothing is known about how lateral root initiation is regulated or coordinated with growth conditions. Here, we report a novel growth assay that allows this regulatory mechanism to be dissected in Arabidopsis. When Arabidopsis seedlings are grown on nutrient media with a high sucrose to nitrogen ratio, lateral root initiation is dramatically repressed. Auxin localization appears to be a key factor in this nutrient-mediated repression of lateral root initiation. We have isolated a mutant, lateral root initiation 1 (lin1), that overcomes the repressive conditions. This mutant produces a highly branched root system on media with high sucrose to nitrogen ratios. The lin1 phenotype is specific to these growth conditions, suggesting that the lin1 gene is involved in coordinating lateral root initiation with nutritional cues. Therefore, these studies provide novel insights into the mechanisms that regulate the earliest steps in lateral root initiation and that coordinate plant development with the environment.
Figures



References
-
- Atkinson D, Hooker J. Using roots in sustainable agriculture. Chem Ind. 1993;4:14–17.
-
- Beeckman T, Burssens S, Inze D. The peri-cell-cycle in Arabidopsis. J Exp Bot. 2001;52:403–411. - PubMed
-
- Berlath T, Sachs T. Plant morphogenesis: long distance coordination and local patterning. Curr Opin Plant Biol. 2001;4:57–62. - PubMed
-
- Boxall S, Martin T, Graham IA. A new class of Arabidopsis mutants that are carbohydrate insensitive. Seventh International Conference on Arabidopsis Research (abstract no. S96). UK: Norwich; 1996.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
