Inhibition of plastocyanin to P(700)(+) electron transfer in Chlamydomonas reinhardtii by hyperosmotic stress
- PMID: 11706196
- PMCID: PMC129285
Inhibition of plastocyanin to P(700)(+) electron transfer in Chlamydomonas reinhardtii by hyperosmotic stress
Abstract
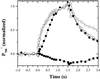
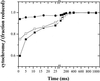
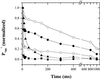
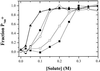
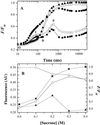
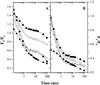
Oxygen electrode and fluorescence studies demonstrate that linear electron transport in the freshwater alga Chlamydomonas reinhardtii can be completely abolished by abrupt hyperosmotic shock. We show that the most likely primary site of inhibition of electron transfer by hyperosmotic shock is a blockage of electron transfer between plastocyanin (PC) or cytochrome c(6) and P(700). The effects on this reaction were reversible upon dilution of the osmolytes and the stability of plastocyanin or photosystem (PS) I was unaffected. Electron micrographs of osmotically shocked cells showed a significant decrease in the thylakoid lumen volume. Comparison of estimated lumenal width with the x-ray structures of plastocyanin and PS I suggest that lumenal space contracts during HOS so as to hinder the movement of docking to PS I of plastocyanin or cytochrome c(6).
Figures


References
-
- Albertsson P-A. Interaction between the lumenal sides of the thylakoid membrane. FEBS Lett. 1982;149:186–190.
-
- Allen JF, Holmes NG. Electron transport and redox titration. In: Hipkins MF, Baker NR, editors. Photosynthesis: Energy Transduction, A Practical Approach. Oxford: IRL Press Limited; 1986. pp. 103–141.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
