Isolation and characterization of a new peroxiredoxin from poplar sieve tubes that uses either glutaredoxin or thioredoxin as a proton donor
- PMID: 11706208
- PMCID: PMC129297
Isolation and characterization of a new peroxiredoxin from poplar sieve tubes that uses either glutaredoxin or thioredoxin as a proton donor
Abstract
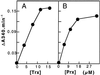
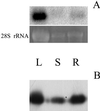
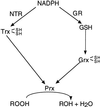
A sequence coding for a peroxiredoxin (Prx) was isolated from a xylem/phloem cDNA library from Populus trichocarpa and subsequently inserted into an expression plasmid yielding the construction pET-Prx. The recombinant protein was produced in Escherichia coli cells and purified to homogeneity with a high yield. The poplar Prx is composed of 162 residues, a property that makes it the shortest plant Prx sequence isolated so far. It was shown that the protein is monomeric and possesses two conserved cysteines (Cys). The Prx degrades hydrogen peroxide and alkyl hydroperoxides in the presence of an exogenous proton donor that can be either thioredoxin or glutaredoxin (Grx). Based on this finding, we propose that the poplar protein represents a new type of Prx that differs from the so-called 2-Cys and 1-Cys Prx, a suggestion supported by the existence of natural fusion sequences constituted of a Prx motif coupled to a Grx motif. The protein was shown to be highly expressed in sieve tubes where thioredoxin h and Grx are also major proteins.
Figures




Similar articles
-
Glutaredoxin-dependent peroxiredoxin from poplar: protein-protein interaction and catalytic mechanism.J Biol Chem. 2002 Apr 19;277(16):13609-14. doi: 10.1074/jbc.M111489200. Epub 2002 Feb 6. J Biol Chem. 2002. PMID: 11832487
-
Engineering functional artificial hybrid proteins between poplar peroxiredoxin II and glutaredoxin or thioredoxin.Biochem Biophys Res Commun. 2006 Mar 24;341(4):1300-8. doi: 10.1016/j.bbrc.2006.01.099. Biochem Biophys Res Commun. 2006. PMID: 16476584
-
Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate.J Biol Chem. 2000 Jul 7;275(27):20346-54. doi: 10.1074/jbc.M001943200. J Biol Chem. 2000. PMID: 10751410
-
The function of peroxiredoxins in plant organelle redox metabolism.J Exp Bot. 2006;57(8):1697-709. doi: 10.1093/jxb/erj160. Epub 2006 Apr 10. J Exp Bot. 2006. PMID: 16606633 Review.
-
Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling.Free Radic Biol Med. 2005 Jun 15;38(12):1543-52. doi: 10.1016/j.freeradbiomed.2005.02.026. Epub 2005 Mar 24. Free Radic Biol Med. 2005. PMID: 15917183 Review.
Cited by
-
Plant glutathione peroxidases are functional peroxiredoxins distributed in several subcellular compartments and regulated during biotic and abiotic stresses.Plant Physiol. 2006 Dec;142(4):1364-79. doi: 10.1104/pp.106.089458. Epub 2006 Oct 27. Plant Physiol. 2006. PMID: 17071643 Free PMC article.
-
Divergent light-, ascorbate-, and oxidative stress-dependent regulation of expression of the peroxiredoxin gene family in Arabidopsis.Plant Physiol. 2003 Jan;131(1):317-25. doi: 10.1104/pp.010017. Plant Physiol. 2003. PMID: 12529539 Free PMC article.
-
Peroxiredoxins in plants and cyanobacteria.Antioxid Redox Signal. 2011 Aug 15;15(4):1129-59. doi: 10.1089/ars.2010.3657. Epub 2011 May 4. Antioxid Redox Signal. 2011. PMID: 21194355 Free PMC article. Review.
-
A mosquito 2-Cys peroxiredoxin protects against nitrosative and oxidative stresses associated with malaria parasite infection.Free Radic Biol Med. 2006 Mar 15;40(6):1067-82. doi: 10.1016/j.freeradbiomed.2005.10.059. Epub 2005 Nov 22. Free Radic Biol Med. 2006. PMID: 16540402 Free PMC article.
-
Functional differentiation of bundle sheath and mesophyll maize chloroplasts determined by comparative proteomics.Plant Cell. 2005 Nov;17(11):3111-40. doi: 10.1105/tpc.105.035519. Epub 2005 Oct 21. Plant Cell. 2005. PMID: 16243905 Free PMC article.
References
-
- Baier M, Dietz KJ. The plant 2-Cys peroxiredoxin BAS1 is a nuclear-encoded chloroplast protein: its expressional regulation, phylogenetic origin, and implications for its specific physiological function in plants. Plant J. 1997;12:179–190. - PubMed
-
- Beckman KB, Ames BN. Oxidative decay of DNA. J Biol Chem. 1997;272:19633–19636. - PubMed
-
- Behm M, Jacquot JP. Isolation and characterization of thioredoxin h from poplar xylem. Plant Physiol Biochem. 2000;38:363–369.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
