Functional and physical interactions of the adaptor protein complex AP-4 with ADP-ribosylation factors (ARFs)
- PMID: 11707398
- PMCID: PMC125733
- DOI: 10.1093/emboj/20.22.6265
Functional and physical interactions of the adaptor protein complex AP-4 with ADP-ribosylation factors (ARFs)
Abstract
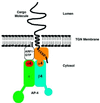
AP-4 is a member of the family of heterotetrameric adaptor protein (AP) complexes that mediate the sorting of integral membrane proteins in post-Golgi compartments. This complex consists of four subunits (epsilon, beta4, mu4 and sigma4) and localizes to the cytoplasmic face of the trans-Golgi network (TGN). Here, we show that the recruitment of endogenous AP-4 to the TGN in vivo is regulated by the small GTP-binding protein ARF1. In addition, we demonstrate a direct interaction of the epsilon and mu4 subunits of AP-4 with ARF1. epsilon binds only to ARF1-GTP and requires residues in the switch I and switch II regions of ARF1. In contrast, mu4 binds equally well to the GTP- and GDP-bound forms of ARF1 and is less dependent on switch I and switch II residues. These observations establish AP-4 as an ARF1 effector and suggest a novel mode of interaction between ARF1 and an AP complex involving both constitutive and regulated interactions.
Figures






References
-
- Aguilar R.C., Ohno,H., Roche,K.W. and Bonifacino,J.S. (1997) Functional domain mapping of the clathrin-associated adaptor medium chains µ1 and µ2. J. Biol. Chem., 272, 27160–27166. - PubMed
-
- Aguilar R.C., Boehm,M., Gorshkova,I., Crouch,R.J., Tomita,K., Saito,T., Ohno,H. and Bonifacino,J.S. (2001) Signal-binding specificity of the µ4 subunit of the adaptor protein complex AP-4. J. Biol. Chem., 276, 13145–13152. - PubMed
-
- Antonny B., Beraud-Dufour,S., Chardin,P. and Chabre,M. (1997) N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry, 36, 4675–4684. - PubMed
-
- Austin C., Hinners,I. and Tooze,S.A. (2000) Direct and GTP-dependent interaction of ADP-ribosylation factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J. Biol. Chem., 275, 21862–21869. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

