Furin initiates gelsolin familial amyloidosis in the Golgi through a defect in Ca(2+) stabilization
- PMID: 11707399
- PMCID: PMC125307
- DOI: 10.1093/emboj/20.22.6277
Furin initiates gelsolin familial amyloidosis in the Golgi through a defect in Ca(2+) stabilization
Abstract
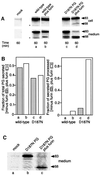
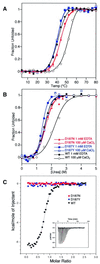
Hereditary familial amyloidosis of Finnish type (FAF) leading to amyloid in the peripheral and central nervous systems stems from deposition of a 71 residue fragment generated from the D187N/Y variants of plasma gelsolin by two sequential endoproteolytic events. We identify the protease accomplishing the first cleavage as furin, a proprotein convertase. Endoproteolysis of plasma gelsolin occurs in the trans-Golgi network due to the inability of the FAF variants to bind and be stabilized by Ca(2+). Secretion and processing of the FAF variants by furin can be uncoupled by blocking the convergence of the exocytic pathway transporting plasma gelsolin and the endocytic recycling of furin. We propose that coincidence of membrane trafficking pathways contributes to the development of proteolysis-initiated amyloid disease.
Figures







References
-
- Aridor M. and Balch,W.E. (1996) Principles of selective transport: coat complexes hold the key. Trends Cell Biol., 6, 315–320. - PubMed
-
- Bennett B.D., Denis,P., Haniu,M., Teplow,D.B., Kahn,S., Louis,J.C., Citron,M. and Vassar,R. (2000) A furin-like convertase mediates propeptide cleavage of BACE, the Alzheimer’s β-secretase. J. Biol. Chem., 275, 37712–37717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

