Proteomic and functional evidence for a P2X7 receptor signalling complex
- PMID: 11707406
- PMCID: PMC125721
- DOI: 10.1093/emboj/20.22.6347
Proteomic and functional evidence for a P2X7 receptor signalling complex
Abstract
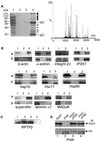
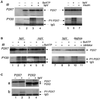
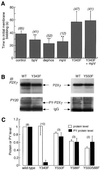
P2X receptors are ATP-gated ion channels in the plasma membrane, but activation of the P2X7 receptor also leads to rapid cytoskeletal re-arrangements such as membrane blebbing. We identified 11 proteins in human embryonic kidney cells that interact with the rat P2X7 receptor, by affinity purification followed by mass spectroscopy and immunoblotting [laminin alpha3, integrin beta2, beta-actin, alpha-actinin, supervillin, MAGuK, three heat shock proteins, phosphatidylinositol 4-kinase and receptor protein tyrosine phosphatase-beta (RPTPbeta)]. Activation of the P2X7 receptor resulted in its dephosphorylation. Whole-cell recordings from cells expressing P2X7 receptors showed that this markedly reduced subsequent ionic currents and it also slowed membrane bleb formation. By mutagenesis, we identified Tyr(343) in the putative second transmembrane domain as the site of phosphorylation. Thus, we have identified a P2X7 receptor signalling complex, some members of which may initiate cytoskeletal rearrangements following receptor activation. Others, such as RPTPbeta, might exert feedback control of the channel itself through its dephosphorylation.
Figures



References
-
- Bronte V., Macino,B., Zambon,A., Rosato,A., Mandruzzato,S., Zanovello,P. and Collavo,D. (1996) Protein tyrosine kinase inhibitors and phosphatases control apoptosis induced by extracellular adenosine 5′-triphosphate. Biochem. Biophys. Res. Commun., 218, 344–351. - PubMed
-
- Caplan A.J. (1999) Hsp90’s secrets unfold: new insights from structural and functional studies. Trends Cell Biol., 9, 262–268. - PubMed
-
- Collo G., Neidhart,S., Kawashima,E., Kosco-Vilbois,M., North,R.A. and Buell,G. (1997) Tissue distribution of the P2X7 receptor. Neuropharmacology, 36, 1277–1283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

