Mutations in the Wolfram syndrome 1 gene (WFS1) are a common cause of low frequency sensorineural hearing loss
- PMID: 11709537
- PMCID: PMC6198816
- DOI: 10.1093/hmg/10.22.2501
Mutations in the Wolfram syndrome 1 gene (WFS1) are a common cause of low frequency sensorineural hearing loss
Abstract
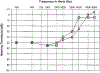
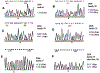
Non-syndromic low frequency sensorineural hearing loss (LFSNHL) affecting only 2000 Hz and below is an unusual type of hearing loss that worsens over time without progressing to profound deafness. This type of LFSNHL may be associated with mild tinnitus but is not associated with vertigo. We have previously reported two families with autosomal dominant LFSNHL linked to adjacent but non-overlapping loci on 4p16, DFNA6 and DFNA14. However, further study revealed that an individual with LFSNHL in the DFNA6 family who had a recombination event that excluded the DFNA14 candidate region was actually a phenocopy, and consequently, DFNA6 and DFNA14 are allelic. LFSNHL appears to be genetically nearly homogeneous, as only one LFSNHL family is known to map to a different chromosome (DFNA1). The DFNA6/14 critical region includes WFS1, the gene responsible for Wolfram syndrome, an autosomal recessive disorder characterized by diabetes mellitus and optic atrophy, and often, deafness. Herein we report five different heterozygous missense mutations (T699M, A716T, V779M, L829P, G831D) in the WFS1 gene found in six LFSNHL families. Mutations in WFS1 were identified in all LFSNHL families tested, with A716T arising independently in two families. None of the mutations was found in at least 220 control chromosomes with the exception of V779M, which was identified in 1/336 controls. This frequency is consistent with the prevalence of heterozygous carriers for Wolfram syndrome estimated at 0.3-1%. An increased risk of sensorineural hearing loss has been reported in such carriers. Therefore, we conclude that mutations in WFS1 are a common cause of LFSNHL.
Figures



References
-
- Vanderbilt Hereditary Deafness Study Group (1968) Dominantly inherited low-frequency hearing loss. Arch. Otolaryngol, 88, 242–250. - PubMed
-
- Kunst H, Marres H. Huygen P, Van Camp G, Joosten F and Cremers C. (1999) Autosomal dominant non-syndromal low-frequency sensorineural hearing impairment linked to chromosome 4p 16 (DFNA14): statistical analysis of hearing threshold in relation to age and evaluation of vestibulo-ocular functions. Audiology, 38, 165–173. - PubMed
-
- Lesperance MM, Hall JW, Bess FH, Fukushima K, Jain PK, Ploplis B, San Agustin TB, Skarka H, Smith RJ, Wills M et al. (1995) A gene for autosomal dominant nonsyndromic hereditary hearing impairment maps to 4p 16.3. Hum. Mol. Genet, 4, 1967–1972. - PubMed
-
- Lynch ED, Lee MK, Morrow JE, Welcsh PL, Leon PE and King MC (1997) Nonsyndromic deafness DFNA1 associated with mutation of a human homolog of the Drosophila gene diaphanous. Science, 278 1315–1318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

