TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3
- PMID: 11711431
- PMCID: PMC312830
- DOI: 10.1101/gad.925901
TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3
Abstract
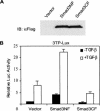
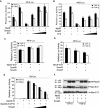
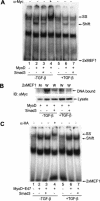
Transforming growth factor-beta (TGF-beta) is a potent inhibitor of skeletal muscle differentiation, but the molecular mechanism and signaling events that lead to this inhibition are poorly characterized. Here we show that the TGF-beta intracellular effector Smad3, but not Smad2, mediates the inhibition of myogenic differentiation in MyoD-expressing C3H10T1/2 cells and C2C12 myoblasts by repressing the activity of the MyoD family of transcriptional factors. The Smad3-mediated repression was directed at the E-box sequence motif within muscle gene enhancers and the bHLH region of MyoD, the domain required for its association with E-protein partners such as E12 and E47. The repression could be overcome by supplying an excess of E12, and covalent tethering of E47 to MyoD rendered the E-box-dependent transcriptional activity refractory to the effects of Smad3 and TGF-beta. Smad3 physically interacted with the HLH domain of MyoD, and this interaction correlated with the ability of Smad3 to interfere with MyoD/E protein heterodimerization and binding of MyoD complexes to oligomerized E-box sites. Together, these results reveal a model for how TGF-beta, through Smad3-mediated transcriptional repression, inhibits myogenic differentiation.
Figures





Similar articles
-
TGF-beta-activated Smad3 represses MEF2-dependent transcription in myogenic differentiation.EMBO J. 2004 Apr 7;23(7):1557-66. doi: 10.1038/sj.emboj.7600179. Epub 2004 Mar 25. EMBO J. 2004. PMID: 15044954 Free PMC article.
-
Myostatin inhibits myoblast differentiation by down-regulating MyoD expression.J Biol Chem. 2002 Dec 20;277(51):49831-40. doi: 10.1074/jbc.M204291200. Epub 2002 Sep 18. J Biol Chem. 2002. PMID: 12244043
-
Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function.J Biol Chem. 2003 Mar 14;278(11):9609-19. doi: 10.1074/jbc.M212259200. Epub 2003 Jan 9. J Biol Chem. 2003. PMID: 12524424
-
Oncogenic mechanisms of Evi-1 protein.Cancer Chemother Pharmacol. 2001 Aug;48 Suppl 1:S35-40. doi: 10.1007/s002800100303. Cancer Chemother Pharmacol. 2001. PMID: 11587364 Review.
-
Murine models define the role of TGF-beta as a master regulator of immune cell function.Cytokine Growth Factor Rev. 2000 Mar-Jun;11(1-2):81-7. doi: 10.1016/s1359-6101(99)00031-3. Cytokine Growth Factor Rev. 2000. PMID: 10708955 Review.
Cited by
-
SMAD3 negatively regulates serum irisin and skeletal muscle FNDC5 and peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) during exercise.J Biol Chem. 2015 Mar 20;290(12):7671-84. doi: 10.1074/jbc.M114.617399. Epub 2015 Feb 3. J Biol Chem. 2015. PMID: 25648888 Free PMC article.
-
Smad3 binds Scleraxis and Mohawk and regulates tendon matrix organization.J Orthop Res. 2013 Sep;31(9):1475-83. doi: 10.1002/jor.22382. Epub 2013 May 7. J Orthop Res. 2013. PMID: 23653374 Free PMC article.
-
Butein is a novel anti-adipogenic compound.J Lipid Res. 2013 May;54(5):1385-96. doi: 10.1194/jlr.M035576. Epub 2013 Mar 6. J Lipid Res. 2013. PMID: 23468131 Free PMC article.
-
TGFbeta1 regulation of vimentin gene expression during differentiation of the C2C12 skeletal myogenic cell line requires Smads, AP-1 and Sp1 family members.Biochim Biophys Acta. 2007 Mar;1773(3):427-39. doi: 10.1016/j.bbamcr.2006.11.017. Epub 2006 Dec 6. Biochim Biophys Acta. 2007. PMID: 17270292 Free PMC article.
-
Micro-Ribonucleic Acid-216a Regulates Bovine Primary Muscle Cells Proliferation and Differentiation via Targeting SMAD Nuclear Interacting Protein-1 and Smad7.Front Genet. 2019 Nov 13;10:1112. doi: 10.3389/fgene.2019.01112. eCollection 2019. Front Genet. 2019. PMID: 31798627 Free PMC article.
References
-
- Bailey P, Downes M, Lau P, Harris J, Chen SL, Hamamori Y, Sartorelli V, Muscat GE. The nuclear receptor corepressor N-CoR regulates differentiation: N-CoR directly interacts with MyoD. Mol Endocrinol. 1999;13:1155–1168. - PubMed
-
- Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: A negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. - PubMed
-
- Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Bi. 1998;14:167–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
