Low threshold T-type calcium current in rat embryonic chromaffin cells
- PMID: 11711559
- PMCID: PMC2278937
- DOI: 10.1111/j.1469-7793.2001.0035k.x
Low threshold T-type calcium current in rat embryonic chromaffin cells
Abstract
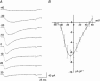
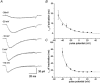
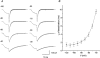
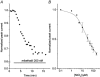
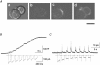
1. The gating kinetics and functions of low threshold T-type current in cultured chromaffin cells from rats of 19-20 days gestation (E19-E20) were studied using the patch clamp technique. Exocytosis induced by calcium currents was monitored by the measurement of membrane capacitance and amperometry with a carbon fibre sensor. 2. In cells cultured for 1-4 days, the embryonic chromaffin cells were immunohistochemically identified by using polyclonal antibodies against dopamine beta-hydroxylase (DBH) and syntaxin. The immuno-positive cells could be separated into three types, based on the recorded calcium current properties. Type I cells showed exclusively large low threshold T-type current, Type II cells showed only high voltage activated (HVA) calcium channel current and Type III cells showed both T-type and HVA currents. These cells represented 44 %, 46 % and 10 % of the total, respectively. 3. T-type current recorded in Type I cells became detectable at -50 mV, reached its maximum amplitude of 6.8 +/- 1.2 pA pF(-1) (n = 5) at -10 mV and reversed around +50 mV. The current was characterized by criss-crossing kinetics within the -50 to -30 mV voltage range and a slow deactivation (deactivation time constant, tau(d) = 2 ms at -80 mV). The channel closing and inactivation process included both voltage-dependent and voltage-independent steps. The antihypertensive drug mibefradil (200 nM) reduced the current amplitude to about 65 % of control values. Ni(2+) also blocked the current in a dose-dependent manner with an IC(50) of 25 microM. 4. T-type current in Type I cells did not induce exocytosis, while catecholamine secretion by exocytosis could be induced by HVA calcium current in both Type II and Type III cells. The failure to induce exocytosis by T-type current in Type I cells was not due to insufficient Ca(2+) influx through the T-type calcium channel. 5. We suggest that T-type current is expressed in developing immature chromaffin cells. The T-type current is replaced progressively by HVA calcium current during pre- and post-natal development accompanying the functional maturation of the exocytosis mechanism.
Figures
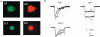



References
-
- Artalejo CR, Adams ME, Fox AP. Three types of Ca2+ channel trigger secretion with different efficacies in chromaffin cells. Nature. 1994;367:72–76. - PubMed
-
- Artalejo CR, Perlman RL, Fox AP. ω-conotoxin GVIA blocks a Ca2+ current in chromaffin cells that is not of the “classic” N type. Neuron. 1992;8:85–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

