Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus
- PMID: 11711570
- PMCID: PMC2278924
- DOI: 10.1111/j.1469-7793.2001.0161k.x
Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus
Abstract
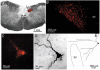
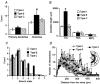
1. The cellular properties of pre-autonomic neurones in the hypothalamic paraventricular nucleus (PVN) were characterized by combining in vivo retrograde tracing techniques, in vitro patch-clamp recordings and three-dimensional reconstruction of recorded neurones in adult hypothalamic slices. 2. The results showed that PVN pre-autonomic neurones constitute a heterogeneous neuronal population. Based on morphological criteria, neurones were classified into three subgroups. Type A neurones (52 %) were located in the ventral parvocellular (PaV) subnucleus, and showed an oblique orientation with respect to the third ventricle (3V). Type B neurones (25 %) were located in the posterior parvocellular (PaPo) subnucleus, and were oriented perpendicularly with respect to the 3V. Type C neurones (23 %) were located in both the PaPo (82 %) and the PaV (18 %) subnuclei, and displayed a concentric dendritic configuration. 3. A morphometric analysis revealed significant differences in the dendritic configuration among neuronal types. Type B neurones had the most complex dendritic arborization, with longer and more branching dendritic trees. 4. Several electrophysiological properties, including cell input resistance and action potential waveforms, differed between cell types, suggesting that the expression and/or properties of a variety of ion channels differ between neuronal types. 5. Common features of PVN pre-autonomic neurones included the expression of a low-threshold spike and strong inward rectification. These properties distinguished them from neighbouring magnocellular vasopressin neurones. 6. In summary, these results indicate that PVN pre-autonomic neurones constitute a heterogeneous neuronal population, and provide a cellular basis for the study of their involvement in the pathophysiology of hypertension and congestive heart failure disorders.
Figures








References
-
- Armstrong WE, Warach S, Hatton G, McNeill T. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5:1931–1958. - PubMed
-
- Badoer E, Oldfield BJ, McKinley MJ. Haemorrhage-induced production of Fos in neurons of the lamina terminalis: role of endogenous angiotensin II. Neuroscience Letters. 1993;159:151–154. - PubMed
-
- Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. American Journal of Physiology. 1995;268:R625–633. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

