Distribution of pacemaker function through the tunica muscularis of the canine gastric antrum
- PMID: 11711577
- PMCID: PMC2278930
- DOI: 10.1111/j.1469-7793.2001.0237k.x
Distribution of pacemaker function through the tunica muscularis of the canine gastric antrum
Abstract
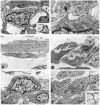
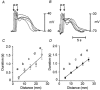
1. Interstitial cells of Cajal (ICC) have been shown to generate pacemaker activity in gastrointestinal (GI) muscles. Experiments were performed to characterize the ICC within the canine gastric antrum and to determine the site(s) of pacemaker activity and whether active propagation pathways exist within the thick-walled tunica muscularis of large mammals. 2. Immunohistochemistry and electron microscopy revealed four populations of ICC within the antral muscularis on the basis of anatomical location. Typical ICC were found in the myenteric region of the small intestine (IC-MY). Intramuscular ICC (IC-IM) were intermingled between muscle fibres of circular and longitudinal muscle layers. ICC were also found within septa (IC-SEP) between muscle bundles and along the submucosal surface of the circular muscle layer (IC-SM). ICC were identified in each location by ultrastructural features. 3. Intracellular electrical recordings demonstrated nifedipine-insensitive slow waves throughout the circular muscle layer. Separation of interior and submucosal circular muscle strips from the dominant (myenteric) pacemaker region dramatically slowed frequency but did not block spontaneous slow waves, suggesting that pacemaker cells populate all regions of the circular muscle. 4. Slow waves could be evoked in interior and submucosal circular muscles at rates above normal antral frequency by electrical pacing or by acetylcholine (0.3 microM). Active slow wave propagation occurred in all regions of the circular muscle, and propagation velocities were similar in each region. 5. In summary, antral muscles of the canine stomach have pacemaker capability throughout the circular muscle. Normally, a dominant pacemaker near the myenteric plexus drives slow waves that actively propagate throughout the circular layer. Pacemaker activity and the active propagation pathway may occur in networks of ICC that are distributed in the region of the myenteric plexus and throughout the circular muscle layer.
Figures








Comment in
-
An additional role for ICC in the control of gastrointestinal motility?J Physiol. 2001 Nov 15;537(Pt 1):1. doi: 10.1111/j.1469-7793.2001.0001k.x. J Physiol. 2001. PMID: 11711554 Free PMC article. No abstract available.
Similar articles
-
Pacing of interstitial cells of Cajal in the murine gastric antrum: neurally mediated and direct stimulation.J Physiol. 2003 Dec 1;553(Pt 2):545-59. doi: 10.1113/jphysiol.2003.050419. Epub 2003 Sep 18. J Physiol. 2003. PMID: 14500772 Free PMC article.
-
Enteric motor neurons form synaptic-like junctions with interstitial cells of Cajal in the canine gastric antrum.Cell Tissue Res. 2003 Mar;311(3):299-313. doi: 10.1007/s00441-002-0657-1. Epub 2003 Feb 25. Cell Tissue Res. 2003. PMID: 12658438
-
Propagation of slow waves in the guinea-pig gastric antrum.J Physiol. 2006 Feb 15;571(Pt 1):165-77. doi: 10.1113/jphysiol.2005.100735. Epub 2005 Dec 15. J Physiol. 2006. PMID: 16357017 Free PMC article.
-
The interstitial cells of Cajal and a gastroenteric pacemaker system.Arch Histol Cytol. 2002 Mar;65(1):1-26. doi: 10.1679/aohc.65.1. Arch Histol Cytol. 2002. PMID: 12002607 Review.
-
The functional role of intramuscular interstitial cells of Cajal in the stomach.J Smooth Muscle Res. 2011;47(2):47-53. doi: 10.1540/jsmr.47.47. J Smooth Muscle Res. 2011. PMID: 21757854 Review.
Cited by
-
Interstitial cells of Cajal in the cynomolgus monkey rectoanal region and their relationship to sympathetic and nitrergic nerves.Am J Physiol Gastrointest Liver Physiol. 2010 May;298(5):G643-56. doi: 10.1152/ajpgi.00260.2009. Epub 2010 Feb 11. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20150245 Free PMC article.
-
Interstitial cells in the primate gastrointestinal tract.Cell Tissue Res. 2012 Nov;350(2):199-213. doi: 10.1007/s00441-012-1468-7. Epub 2012 Aug 3. Cell Tissue Res. 2012. PMID: 22864981 Free PMC article.
-
Pacing of interstitial cells of Cajal in the murine gastric antrum: neurally mediated and direct stimulation.J Physiol. 2003 Dec 1;553(Pt 2):545-59. doi: 10.1113/jphysiol.2003.050419. Epub 2003 Sep 18. J Physiol. 2003. PMID: 14500772 Free PMC article.
-
The significance of interstitial cells in neurogastroenterology.J Neurogastroenterol Motil. 2014 Jul 31;20(3):294-317. doi: 10.5056/jnm14060. J Neurogastroenterol Motil. 2014. PMID: 24948131 Free PMC article.
-
Interstitial cells of Cajal in the normal human gut and in Hirschsprung disease.Pediatr Surg Int. 2013 Sep;29(9):889-97. doi: 10.1007/s00383-013-3364-y. Pediatr Surg Int. 2013. PMID: 23917331 Review.
References
-
- Bauer AJ, Publicover NG, Sanders KM. Origin and spread of slow waves in canine gastric antral circular muscle. American Journal of Physiology. 1985a;249:G800–806. - PubMed
-
- Bauer AJ, Sanders KM. Passive and active membrane properties of canine gastric antral circular muscles. American Journal of Physiology. 1986;251:C268–273. - PubMed
-
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell and Tissue Research. 1997;290:11–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

