Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection
- PMID: 11711588
- PMCID: PMC116093
- DOI: 10.1128/JVI.75.24.11983-11991.2001
Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection
Abstract
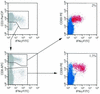
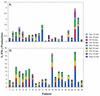
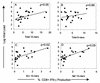
Human immunodeficiency virus (HIV)-specific T-cell responses are thought to play a key role in viral load decline during primary infection and in determining the subsequent viral load set point. The requirements for this effect are unknown, partly because comprehensive analysis of total HIV-specific CD4(+) and CD8(+) T-cell responses to all HIV-encoded epitopes has not been accomplished. To assess these responses, we used cytokine flow cytometry and overlapping peptide pools encompassing all products of the HIV-1 genome to study total HIV-specific T-cell responses in 23 highly active antiretroviral therapy naïve HIV-infected patients. HIV-specific CD8(+) T-cell responses were detectable in all patients, ranging between 1.6 and 18.4% of total CD8(+) T cells. HIV-specific CD4(+) T-cell responses were present in 21 of 23 patients, although the responses were lower (0.2 to 2.94%). Contrary to previous reports, a positive correlation was identified between the plasma viral load and the total HIV-, Env-, and Nef-specific CD8(+) T-cell frequency. No correlation was found either between viral load and total or Gag-specific CD4(+) T-cell response or between the frequency of HIV-specific CD4(+) and CD8(+) T cells. These results suggest that overall frequencies of HIV-specific T cells are not the sole determinant of immune-mediated protection in HIV-infection.
Figures


References
-
- Allen T M, O'Connor D H, Jing P, Dzuris J L, Mothe B R, Vogel T U, Dunphy E, Liebl M E, Emerson C, Wilson N, Kunstman K J, Wang X, Allison D B, Hughes A L, Desrosiers R C, Altman J D, Wolinsky S M, Sette A, Watkins D I. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature. 2000;407:386–390. - PubMed
-
- Altfeld M, Rosenberg E S, Shankarappa R, Mukherjee J S, Hecht F M, Eldridge R L, Addo M M, Poon S H, Phillips M N, Robbins G K, Sax P E, Boswell S, Kahn J O, Brander C, Goulder P J, Levy J A, Mullins J I, Walker B D. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001;193:169–180. - PMC - PubMed
-
- Amara R R, Villinger F, Altman J, Lydy S L, O'Neil S P, Staprans S I, Montefiori D C, Xu Y, Herndon J G, Wyatt L S, Candido M A, Kozyr N L, Earl P L, Smith J M, Ma H-L, Grimm B D, Hulsey M L, Miller J, McClure H M, McNicholl J M, Moss B, Robinson H L. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. - PubMed
-
- Appay V, Nixon D F, Donahoe S M, Gillespie G M, Dong T, King A, Ogg G S, Spiegel H M, Conlon C, Spina C A, Havlir D V, Richman D D, Waters A, Easterbrook P, McMichael A J, Rowland-Jones S L. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. - PMC - PubMed
-
- Barouch D H, Santra S, Schmitz J E, Kuroda M J, Fu T M, Wagner W, Bilska M, Craiu A, Zheng X X, Krivulka G R, Beaudry K, Lifton M A, Nickerson C E, Trigona W L, Punt K, Freed D C, Guan L, Dubey S, Casimiro D, Simon A, Davies M E, Chastain M, Strom T B, Gelman R S, Montefiori D C, Lewis M G. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

