Effector function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1
- PMID: 11711607
- PMCID: PMC116112
- DOI: 10.1128/JVI.75.24.12161-12168.2001
Effector function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1
Abstract
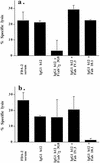
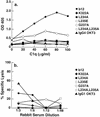
The human antibody immunoglobulin G1 (IgG1) b12 neutralizes a broad range of human immunodeficiency virus-type 1 (HIV-1) isolates in vitro and is able to protect against viral challenge in animal models. Neutralization of free virus, which is an antiviral activity of antibody that generally does not require the antibody Fc fragment, likely plays an important role in the protection observed. The role of Fc-mediated effector functions, which may reduce infection by inducing phagocytosis and lysis of virions and infected cells, however, is less clear. To investigate this role, we constructed a panel of IgG1 b12 mutants with point mutations in the second domain of the antibody heavy chain constant region (CH2). These mutations, as expected, did not affect gp120 binding or HIV-1 neutralization. IgG1 b12 mediated strong antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) of HIV-1-infected cells, but these activities were reduced or abrogated for the antibody mutants. Two mutants were of particular interest. K322A showed a twofold reduction in FcgammaR binding affinity and ADCC, while C1q binding and CDC were abolished. A double mutant (L234A, L235A) did not bind either FcgammaR or C1q, and both ADCC and CDC functions were abolished. In this study, we confirmed that K322 forms part of the C1q binding site in human IgG1 and plays an important role in the molecular interactions leading to complement activation. Less expectedly, we demonstrate that the lower hinge region in human IgG1 has a strong modulating effect on C1q binding and CDC. The b12 mutants K322A and L234A, L235A are useful tools for dissecting the in vivo roles of ADCC and CDC in the anti-HIV-1 activity of neutralizing antibodies.
Figures
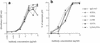

References
-
- Andrus L, Prince A M, Bernal I, McCormack P, Lee D H, Gorny M K, Zolla-Pazner S. Passive immunization with human immunodeficiency virus type 1-neutralizing monoclonal antibody in hu-PBL-SCID mice: isolation of a neutralization escape variant. J Infect Dis. 1998;177:889–897. - PubMed
-
- Baba T W, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini L A, Posner M R, Katinger H, Stiegler G, Bernacky B J, Rizvi T A, Schmidt R, Hill L R, Keeling M E, Lu Y, Wright J E, Chou T C, Ruprecht R M. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. - PubMed
-
- Bebbington C R, Renner G, Thomson S, King D, Abrams D, Yarranton G T. High-level expression of a recombinant antibody from myeloma cells using a glutamine synthetase gene as an amplifiable selectable marker. Bio/Technology. 1992;10:169–175. - PubMed
-
- Bindon C I, Hale G, Waldmann H. Importance of antigen specificity for complement-mediated lysis by monoclonal antibodies. Eur J Immunol. 1988;18:1507–1514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

