Neuropeptide-induced androgen independence in prostate cancer cells: roles of nonreceptor tyrosine kinases Etk/Bmx, Src, and focal adhesion kinase
- PMID: 11713275
- PMCID: PMC100003
- DOI: 10.1128/MCB.21.24.8385-8397.2001
Neuropeptide-induced androgen independence in prostate cancer cells: roles of nonreceptor tyrosine kinases Etk/Bmx, Src, and focal adhesion kinase
Abstract
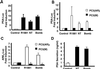
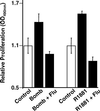
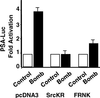
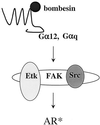
The bombesin/gastrin-releasing peptide (GRP) family of neuropeptides has been implicated in various in vitro and in vivo models of human malignancies including prostate cancers. It was previously shown that bombesin and/or neurotensin (NT) acts as a survival and migratory factor(s) for androgen-independent prostate cancers. However, a role in the transition from an androgen-dependent to -refractory state has not been addressed. In this study, we investigate the biological effects and signal pathways of bombesin and NT on LNCaP, a prostate cancer cell line which requires androgen for growth. We show that both neurotrophic factors can induce LNCaP growth in the absence of androgen. Concurrent transactivation of reporter genes driven by the prostate-specific antigen promoter or a promoter carrying an androgen-responsive element (ARE) indicate that growth stimulation is accompanied by androgen receptor (AR) activation. Furthermore, neurotrophic factor-induced gene activation was also present in PC3 cells transfected with the AR but not in the parental line which lacks the AR. Given that bombesin does not directly bind to the AR and is known to engage a G-protein-coupled receptor, we investigated downstream signaling events that could possibly interact with the AR pathway. We found that three nonreceptor tyrosine kinases, focal adhesion kinase (FAK), Src, and Etk/BMX play important parts in this process. Etk/Bmx activation requires FAK and Src and is critical for neurotrophic factor-induced growth, as LNCaP cells transfected with a dominant-negative Etk/BMX fail to respond to bombesin. Etk's activation requires FAK, Src, but not phosphatidylinositol 3-kinase. Likewise, bombesin-induced AR activation is inhibited by the dominant-negative mutant of either Src or FAK. Thus, in addition to defining a new G-protein pathway, this report makes the following points regarding prostate cancer. (i) Neurotrophic factors can activate the AR, thus circumventing the normal growth inhibition caused by androgen ablation. (ii) Tyrosine kinases are involved in neurotrophic factor-mediated AR activation and, as such, may serve as targets of future therapeutics, to be used in conjunction with current antihormone and antineuropeptide therapies.
Figures
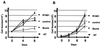


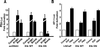

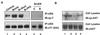

Similar articles
-
Interleukin-8 confers androgen-independent growth and migration of LNCaP: differential effects of tyrosine kinases Src and FAK.Oncogene. 2004 Mar 18;23(12):2197-205. doi: 10.1038/sj.onc.1207344. Oncogene. 2004. PMID: 14767470
-
Angiogenesis is not mediated by prostate cancer neuropeptides.Angiogenesis. 2003;6(4):289-93. doi: 10.1023/B:AGEN.0000029409.94626.64. Angiogenesis. 2003. PMID: 15166497
-
Inappropriate activation of the androgen receptor by nonsteroids: involvement of the Src kinase pathway and its therapeutic implications.Cancer Res. 2006 Nov 1;66(21):10449-59. doi: 10.1158/0008-5472.CAN-06-2582. Cancer Res. 2006. PMID: 17079466
-
Oncogenic activation of androgen receptor.Urol Oncol. 2009 Jan-Feb;27(1):48-52. doi: 10.1016/j.urolonc.2008.06.002. Urol Oncol. 2009. PMID: 19111798 Free PMC article. Review.
-
Gastrointestinal peptide signalling in health and disease.Eur J Surg Suppl. 2002;(587):23-38. Eur J Surg Suppl. 2002. PMID: 16144198 Review.
Cited by
-
Preclinical Models in Prostate Cancer: Resistance to AR Targeting Therapies in Prostate Cancer.Cancers (Basel). 2021 Feb 22;13(4):915. doi: 10.3390/cancers13040915. Cancers (Basel). 2021. PMID: 33671614 Free PMC article. Review.
-
Expression and functional role of orphan receptor GPR158 in prostate cancer growth and progression.PLoS One. 2015 Feb 18;10(2):e0117758. doi: 10.1371/journal.pone.0117758. eCollection 2015. PLoS One. 2015. PMID: 25693195 Free PMC article.
-
Protocadherin-PC promotes androgen-independent prostate cancer cell growth.Prostate. 2006 Jul 1;66(10):1100-13. doi: 10.1002/pros.20446. Prostate. 2006. PMID: 16637074 Free PMC article.
-
Therapeutic targeting of the prostate cancer microenvironment.Nat Rev Urol. 2010 Sep;7(9):494-509. doi: 10.1038/nrurol.2010.134. Nat Rev Urol. 2010. PMID: 20818327 Review.
-
Identification of molecular targets in urologic oncology.World J Urol. 2009 Feb;27(1):3-8. doi: 10.1007/s00345-008-0339-z. Epub 2008 Nov 12. World J Urol. 2009. PMID: 19002690 Review.
References
-
- Abi-Aad A S, Opsomer R J. Prostate cancer–treatment of disseminated disease. Acta Urol Belg. 1996;64:67–76. - PubMed
-
- Abrahamsson P A. Neuroendocrine cells in tumour growth of the prostate. Endocr Relat Cancer. 1999;6:503–519. - PubMed
-
- Abrahamsson P A. Neuroendocrine differentiation in prostatic carcinoma. Prostate. 1999;39:135–148. - PubMed
-
- Abrahamsson P A, Falkmer S, Falt K, Grimelius L. The course of neuroendocrine differentiation in prostatic carcinomas. An immunohistochemical study testing chromogranin A as an “endocrine marker.”. Pathol Res Pract. 1989;185:373–380. - PubMed
-
- Abrahamsson P A, Wadstrom L B, Alumets J, Falkmer S, Grimelius L. Peptide-hormone- and serotonin-immunoreactive tumour cells in carcinoma of the prostate. Pathol Res Pract. 1987;182:298–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
