The putative oncoprotein Bcl-3 induces cyclin D1 to stimulate G(1) transition
- PMID: 11713278
- PMCID: PMC100006
- DOI: 10.1128/MCB.21.24.8428-8436.2001
The putative oncoprotein Bcl-3 induces cyclin D1 to stimulate G(1) transition
Abstract
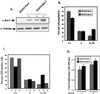
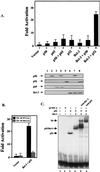
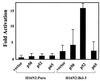
Bcl-3 is a distinctive member of the IkappaB family of NF-kappaB inhibitors because it can function to coactivate transcription. A potential involvement of Bcl-3 in oncogenesis is highlighted by the fact that it was cloned due to its location at a breakpoint junction in some cases of human B-cell chronic lymphocytic leukemia and that it is highly expressed in human breast tumor tissue. To analyze the effects of Bcl-3 dysregulation in breast epithelial cells, we created stable immortalized human breast epithelial cell lines either expressing Bcl-3 or carrying the corresponding vector control plasmid. Analysis of the Bcl-3-expressing cells suggests that these cells have a shortened G(1) phase of the cell cycle as well as a significant increase in hyperphosphorylation of the retinoblastoma protein. Additionally, the cyclin D1 gene was found to be highly expressed in these cells. Upon further analysis, Bcl-3, acting as a coactivator with NF-kappaB p52 homodimers, was demonstrated to directly activate the cyclin D1 promoter through an NF-kappaB binding site. Therefore, our results demonstrate that dysregulated expression of Bcl-3 potentiates the G(1) transition of the cell cycle by stimulating the transcription of the cyclin D1 gene in human breast epithelial cells.
Figures


References
-
- Albanese C, Johnson G, Watanabe N, Eklund N, Vu D, Arnold A, Pestell R G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. - PubMed
-
- Baldwin A S., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. - PubMed
-
- Beijersbergen R L, Bernards R. Cell cycle regulation by the retinoblastoma family of growth inhibitory proteins. Biochim Biophys Acta. 1996;1287:103–120. - PubMed
-
- Bours V, Franzoso G, Azarenko V, Park S, Kanno T, Brown K, Siebenlist U. The oncoprotein Bcl-3 directly transactivates through kappa B motifs via association with DNA-binding p50B homodimers. Cell. 1993;72:729–739. - PubMed
-
- Brasier A R, Lu M, Hai T, Lu Y, Boldogh I. NF-κB inducible BCL-3 expression is an autoregulatory loop controlling nuclear p50/NF-κB1 residence. J Biol Chem. 2001;276:32080–32093. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
