Mice lacking desmocollin 1 show epidermal fragility accompanied by barrier defects and abnormal differentiation
- PMID: 11714727
- PMCID: PMC2150874
- DOI: 10.1083/jcb.200105009
Mice lacking desmocollin 1 show epidermal fragility accompanied by barrier defects and abnormal differentiation
Abstract
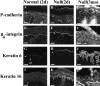
The desmosomal cadherin desmocollin (Dsc)1 is expressed in upper epidermis where strong adhesion is required. To investigate its role in vivo, we have genetically engineered mice with a targeted disruption in the Dsc1 gene. Soon after birth, null mice exhibit flaky skin and a striking punctate epidermal barrier defect. The epidermis is fragile, and acantholysis in the granular layer generates localized lesions, compromising skin barrier function. Neutrophils accumulate in the lesions and further degrade the tissue, causing sloughing (flaking) of lesional epidermis, but rapid wound healing prevents the formation of overt lesions. Null epidermis is hyperproliferative and overexpresses keratins 6 and 16, indicating abnormal differentiation. From 6 wk, null mice develop ulcerating lesions resembling chronic dermatitis. We speculate that ulceration occurs after acantholysis in the fragile epidermis because environmental insults are more stringent and wound healing is less rapid than in neonatal mice. This dermatitis is accompanied by localized hair loss associated with formation of utriculi and dermal cysts, denoting hair follicle degeneration. Possible resemblance of the lesions to human blistering diseases is discussed. These results show that Dsc1 is required for strong adhesion and barrier maintenance in epidermis and contributes to epidermal differentiation.
Figures








Similar articles
-
Suprabasal desmoglein 3 expression in the epidermis of transgenic mice results in hyperproliferation and abnormal differentiation.Mol Cell Biol. 2002 Aug;22(16):5846-58. doi: 10.1128/MCB.22.16.5846-5858.2002. Mol Cell Biol. 2002. PMID: 12138195 Free PMC article.
-
Loss of desmocollin 3 in mice leads to epidermal blistering.J Cell Sci. 2008 Sep 1;121(Pt 17):2844-9. doi: 10.1242/jcs.031518. Epub 2008 Aug 5. J Cell Sci. 2008. PMID: 18682494 Free PMC article.
-
Desmosomal cadherin misexpression alters beta-catenin stability and epidermal differentiation.Mol Cell Biol. 2005 Feb;25(3):969-78. doi: 10.1128/MCB.25.3.969-978.2005. Mol Cell Biol. 2005. PMID: 15657425 Free PMC article.
-
Inherited disorders of the skin in human and mouse: from development to differentiation.Int J Dev Biol. 2004;48(2-3):171-9. doi: 10.1387/ijdb.15272382. Int J Dev Biol. 2004. PMID: 15272382 Review.
-
Analysis of desmosomal cadherin-adhesive function and stoichiometry of desmosomal cadherin-plakoglobin complexes.J Invest Dermatol. 1996 Sep;107(3):293-300. doi: 10.1111/1523-1747.ep12363000. J Invest Dermatol. 1996. PMID: 8751959 Review.
Cited by
-
Cell adhesion in epidermal development and barrier formation.Curr Top Dev Biol. 2015;112:383-414. doi: 10.1016/bs.ctdb.2014.11.027. Epub 2015 Feb 11. Curr Top Dev Biol. 2015. PMID: 25733147 Free PMC article. Review.
-
A complex of cadherin 17 with desmocollin 1 and p120-catenin regulates colorectal cancer migration and invasion according to the cell phenotype.J Exp Clin Cancer Res. 2024 Jan 24;43(1):31. doi: 10.1186/s13046-024-02956-6. J Exp Clin Cancer Res. 2024. PMID: 38263178 Free PMC article.
-
Desmosomes: A light microscopic and ultrastructural analysis of desmosomes in odontogenic cysts.J Oral Maxillofac Pathol. 2014 Sep-Dec;18(3):336-40. doi: 10.4103/0973-029X.151309. J Oral Maxillofac Pathol. 2014. PMID: 25948985 Free PMC article.
-
Structural basis of adhesive binding by desmocollins and desmogleins.Proc Natl Acad Sci U S A. 2016 Jun 28;113(26):7160-5. doi: 10.1073/pnas.1606272113. Epub 2016 Jun 13. Proc Natl Acad Sci U S A. 2016. PMID: 27298358 Free PMC article.
-
The desmosome.Cold Spring Harb Perspect Biol. 2009 Aug;1(2):a002543. doi: 10.1101/cshperspect.a002543. Cold Spring Harb Perspect Biol. 2009. PMID: 20066089 Free PMC article. Review.
References
-
- Amagai, M. 1999. Autoimmunity against desmosomal cadherins in pemphigus. J. Dermatol. Sci. 20:92–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

