More sweetness than light? A search for the causes of diabetic vasculopathy
- PMID: 11714733
- PMCID: PMC209431
- DOI: 10.1172/JCI14508
More sweetness than light? A search for the causes of diabetic vasculopathy
Figures
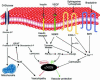
Comment on
-
Nitric oxide synthases: which, where, how, and why?J Clin Invest. 1997 Nov 1;100(9):2146-52. doi: 10.1172/JCI119750. J Clin Invest. 1997. PMID: 9410890 Free PMC article. Review. No abstract available.
-
Cellular and molecular mechanisms of endothelial cell dysfunction.J Clin Invest. 1997 Nov 1;100(9):2153-7. doi: 10.1172/JCI119751. J Clin Invest. 1997. PMID: 9410891 Free PMC article. Review. No abstract available.
-
Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site.J Clin Invest. 2001 Nov;108(9):1341-8. doi: 10.1172/JCI11235. J Clin Invest. 2001. PMID: 11696579 Free PMC article.
References
-
- McKinlay J, Marceau L. US public health and the 21st century: diabetes mellitus. Lancet. 2000;356:757–761. - PubMed
-
- Loscalzo J, Welch G. Nitric oxide and its role in the cardiovascular system. Prog Cardiovasc Dis. 1995;38:87–104. - PubMed
-
- Hart GW. Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu Rev Biochem. 1997;66:315–335. - PubMed

