HIV-1 Vpr enhances viral burden by facilitating infection of tissue macrophages but not nondividing CD4+ T cells
- PMID: 11714748
- PMCID: PMC2193684
- DOI: 10.1084/jem.194.10.1407
HIV-1 Vpr enhances viral burden by facilitating infection of tissue macrophages but not nondividing CD4+ T cells
Abstract
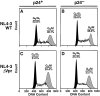
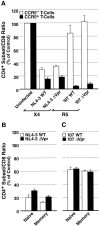
Prior experiments in explants of human lymphoid tissue have demonstrated that human immunodeficiency virus type 1 (HIV-1) productively infects diverse cellular targets including T cells and tissue macrophages. We sought to determine the specific contribution of macrophages and T cells to the overall viral burden within lymphoid tissue. To block infection of macrophages selectively while preserving infection of T cells, we used viruses deficient for viral protein R (Vpr) that exhibit profound replication defects in nondividing cells in vitro. We inoculated tonsil histocultures with matched pairs of congenic viruses that differed only by the presence of a wild-type or truncated vpr gene. Although these viruses exhibited no reduction in the infection or depletion of T cells, the ability of the Vpr-deficient R5 virus to infect tissue macrophages was severely impaired compared with matched wild-type R5 virus. Interestingly, the Vpr-deficient R5 virus also exhibited a 50% reduction in overall virus replication compared with its wild-type counterpart despite the fact that macrophages represent a small fraction of the potential targets of HIV-1 infection in these tissues. Collectively, these data highlight the importance of tissue macrophages in local viral burden and further implicate roles for CC chemokine receptor 5, macrophages, and Vpr in the life cycle and pathogenesis of HIV-1.
Figures
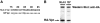
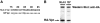
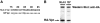



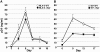
References
-
- Balliet, J.W., D.L. Kolson, G. Eiger, F.M. Kim, K.A. McGann, A. Srinivasan, and R. Collman. 1994. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology. 200:623–631. - PubMed
-
- Heinzinger, N.K., M.I. Bukinsky, S.A. Haggerty, A.M. Ragland, V. Kewalramani, M.A. Lee, H.E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA. 91:7311–7315. - PMC - PubMed
-
- Connor, R.I., B.K. Chen, S. Choe, and N.R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 206:935–944. - PubMed

