Crystal structure of calcium-free human sorcin: a member of the penta-EF-hand protein family
- PMID: 11714909
- PMCID: PMC2374028
- DOI: 10.1110/ps.36701
Crystal structure of calcium-free human sorcin: a member of the penta-EF-hand protein family
Abstract
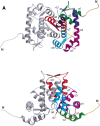
Sorcin is a 22 kD calcium-binding protein that is found in a wide variety of cell types, such as heart, muscle, brain and adrenal medulla. It belongs to the penta-EF-hand (PEF) protein family, which contains five EF-hand motifs that associate with membranes in a calcium-dependent manner. Prototypic members of this family are the calcium-binding domains of calpain, such as calpain dVI. Full-length human sorcin has been crystallized in the absence of calcium and the structure determined at 2.2 A resolution. Apart from an extended N-terminal portion, the sorcin molecule has a globular shape. The C-terminal domain is predominantly alpha-helical, containing eight alpha-helices and connecting loops incorporating five EF hands. Sorcin forms dimers through the association of the unpaired EF5, confirming this as the mode of association in the dimerization of PEF proteins. Comparison with calpain dVI reveals that the general folds of the individual EF-hand motifs are conserved, especially that of EF1, the novel EF-hand motif characteristic of the family. Detailed structural comparisons of sorcin with other members of PEF indicate that the EF-hand pair EF1-EF2 is likely to correspond to the two physiologically relevant calcium-binding sites and that the calcium-induced conformational change may be modest and localized within this pair of EF-hands. Overall, the results derived from the structural observations support the view that, in sorcin, calcium signaling takes place through the first pair of EF-hands.
Figures


Similar articles
-
Crystal structure of human grancalcin, a member of the penta-EF-hand protein family.J Mol Biol. 2000 Jul 28;300(5):1271-81. doi: 10.1006/jmbi.2000.3925. J Mol Biol. 2000. PMID: 10903868
-
The crystal structure of the sorcin calcium binding domain provides a model of Ca2+-dependent processes in the full-length protein.J Mol Biol. 2002 Mar 29;317(3):447-58. doi: 10.1006/jmbi.2002.5417. J Mol Biol. 2002. PMID: 11922676
-
Structure-function relationships in sorcin, a member of the penta EF-hand family. Interaction of sorcin fragments with the ryanodine receptor and an Escherichia coli model system.Biochemistry. 2000 Feb 1;39(4):658-66. doi: 10.1021/bi991648v. Biochemistry. 2000. PMID: 10651630
-
Structures, functions and molecular evolution of the penta-EF-hand Ca2+-binding proteins.Biochim Biophys Acta. 2002 Nov 4;1600(1-2):51-60. doi: 10.1016/s1570-9639(02)00444-2. Biochim Biophys Acta. 2002. PMID: 12445459 Review.
-
Structure and function of ALG-2, a penta-EF-hand calcium-dependent adaptor protein.Sci China Life Sci. 2011 Aug;54(8):770-9. doi: 10.1007/s11427-011-4204-8. Epub 2011 Jul 24. Sci China Life Sci. 2011. PMID: 21786200 Review.
Cited by
-
Sorcin, a potential therapeutic target for reversing multidrug resistance in cancer.J Physiol Biochem. 2012 Jun;68(2):281-7. doi: 10.1007/s13105-011-0140-0. J Physiol Biochem. 2012. PMID: 22701893 Review. No abstract available.
-
Sorcin regulate pyroptosis by interacting with NLRP3 inflammasomes to facilitate the progression of hepatocellular carcinoma.Cell Death Dis. 2023 Oct 13;14(10):678. doi: 10.1038/s41419-023-06096-1. Cell Death Dis. 2023. PMID: 37833249 Free PMC article.
-
Calretinin and calbindin D28k have different domain organizations.Protein Sci. 2003 Jan;12(1):180-4. doi: 10.1110/ps.0215303. Protein Sci. 2003. PMID: 12493841 Free PMC article.
-
Identification of a novel Sorcin isoform with a different C-terminal but intact dimerization property.Sci Rep. 2023 Sep 14;13(1):15262. doi: 10.1038/s41598-023-40913-z. Sci Rep. 2023. PMID: 37709787 Free PMC article.
-
A technique for high-throughput protein crystallization in ionically cross-linked polysaccharide gel beads for X-ray diffraction experiments.PLoS One. 2014 Apr 16;9(4):e95017. doi: 10.1371/journal.pone.0095017. eCollection 2014. PLoS One. 2014. PMID: 24740192 Free PMC article.
References
-
- Blanchard, H., P. Grochulski, Y. Li, J.S. Arthur, P.L. Davies, J.S. Elce, and M. Cygler. 1997. Structure of a calpain Ca(2+)-binding domain reveals a novel EF-hand and Ca(2+)-induced conformational changes. Nat. Struct. Biol. 4 532–538. - PubMed
-
- Boyhan, A., C.M. Casimir, J.K. French, C.G. Teahan, and A.W. Segal. 1992. Molecular cloning and characterization of grancalcin, a novel EF-hand calcium-binding protein abundant in neutrophils and monocytes. J. Biol. Chem.. 267 2928–2933. - PubMed
-
- Brownawell, A.M., and C.E. Creutz. 1997. Calcium-dependent binding of sorcin to the N-terminal domain of synexin (annexin VII). J. Biol. Chem.. 272 22182–22190. - PubMed
-
- Brunger, A.T., P.D. Adams, G.M. Clore, W.L. DeLano, P. Gros, R.W. Grosse-Kunstleve, J.S. Jiang, J. Kuszewski, M. Nilges, N.S. Pannu, R.J. Read, L.M. Rice, T. Simonson, and G.L. Warren. 1998. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 54 905–921. - PubMed
-
- Graham-Siegenthaler, K., S. Gauthier, P.L. Davies, and J.S. Elce. 1994. Active recombinant rat calpain II. Bacterially produced large and small subunits associate both in vivo and in vitro. J Biol Chem. 269 30457–30460. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

