Identification of a region in G protein gamma subunits conserved across species but hypervariable among subunit isoforms
- PMID: 11714923
- PMCID: PMC2374038
- DOI: 10.1110/ps.ps.26401
Identification of a region in G protein gamma subunits conserved across species but hypervariable among subunit isoforms
Abstract
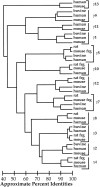
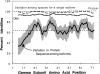
The heterotrimeric GTP binding proteins, G proteins, consist of three distinct subunits: alpha, beta, and gamma. There are 12 known mammalian gamma subunit genes whose products are the smallest and most variable of the G protein subunits. Sequencing of the bovine brain gamma(10) protein by electrospray mass spectrometry revealed that it differs from the human protein by an Ala to Val substitution near the N-terminus. Comparison of gamma isoform subunit sequences indicated that they vary substantially more at the N-terminus than at other parts of the protein. Thus, species variation of this region might reflect the lack of conservation of a functionally unimportant part of the protein. Analysis of 38 gamma subunit sequences from four different species shows that the N-terminus of a given gamma subunit isoform is as conserved between different species as any other part of the protein, including highly conserved regions. These data suggest that the N-terminus of gamma is a functionally important part of the protein exhibiting substantial isoform-specific variation.
Figures

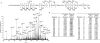
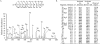

Similar articles
-
Gamma 2 subunit of G protein heterotrimer is an N-end rule ubiquitylation substrate.Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):5081-6. doi: 10.1073/pnas.0831228100. Epub 2003 Apr 16. Proc Natl Acad Sci U S A. 2003. PMID: 12700354 Free PMC article.
-
Structural characterization of intact G protein gamma subunits by mass spectrometry.Methods Enzymol. 2002;344:586-97. doi: 10.1016/s0076-6879(02)44742-8. Methods Enzymol. 2002. PMID: 11771413 No abstract available.
-
Heterogeneous processing of a G protein gamma subunit at a site critical for protein and membrane interactions.Biochemistry. 1998 Sep 1;37(35):12280-6. doi: 10.1021/bi980230e. Biochemistry. 1998. PMID: 9724542
-
Comprehensive mass spectrometric analysis of the 20S proteasome complex.Methods Enzymol. 2005;405:187-236. doi: 10.1016/S0076-6879(05)05009-3. Methods Enzymol. 2005. PMID: 16413316 Review.
-
Heterotrimeric G-proteins: a short history.Br J Pharmacol. 2006 Jan;147 Suppl 1(Suppl 1):S46-55. doi: 10.1038/sj.bjp.0706405. Br J Pharmacol. 2006. PMID: 16402120 Free PMC article. Review.
Cited by
-
Heterotrimeric G-proteins in Picea abies and their regulation in response to Heterobasidion annosum s.l. infection.BMC Plant Biol. 2015 Dec 12;15:287. doi: 10.1186/s12870-015-0676-1. BMC Plant Biol. 2015. PMID: 26654722 Free PMC article.
-
Subtype-dependent regulation of Gβγ signalling.Cell Signal. 2021 Jun;82:109947. doi: 10.1016/j.cellsig.2021.109947. Epub 2021 Feb 11. Cell Signal. 2021. PMID: 33582184 Free PMC article. Review.
-
Central and C-terminal domains of heterotrimeric G protein gamma subunits differentially influence the signaling necessary for primordial germ cell migration.Cell Signal. 2011 Oct;23(10):1617-24. doi: 10.1016/j.cellsig.2011.05.015. Epub 2011 Jun 15. Cell Signal. 2011. PMID: 21699975 Free PMC article.
-
Gγ and Gα Identity Dictate a G-Protein Heterotrimer Plasma Membrane Targeting.Cells. 2019 Oct 13;8(10):1246. doi: 10.3390/cells8101246. Cells. 2019. PMID: 31614907 Free PMC article.
-
Gγ identity dictates efficacy of Gβγ signaling and macrophage migration.J Biol Chem. 2018 Feb 23;293(8):2974-2989. doi: 10.1074/jbc.RA117.000872. Epub 2018 Jan 9. J Biol Chem. 2018. PMID: 29317505 Free PMC article.
References
-
- Bokoch, G.M., Katada, T., Northup, J.K., Ui, M., and Gilman, A.G. 1984. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J. Biol. Chem. 259 3560–3567. - PubMed
-
- Clarke, S. 1992. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu. Rev. Biochem. 61 355–386. - PubMed
-
- Cook, L.A., Schey, K.L., Wilcox, M.D., Dingus, J., and Hildebrandt, J.D. 1998. Heterogeneous C-terminal processing of a G protein gamma subunit. Biochemistry 37 12280–12286. - PubMed
-
- Cook, L.A., Wilcox, M.D., Dingus, J., Schey, K.L., and Hildebrandt, J.D. Separation and analysis of G protein γ subunits. Methods Enzymol. (in press). - PubMed
-
- Cox, A.D. 1995. Mutation and analysis of prenylation signal sequences. Methods Enzymol. 250 105–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

