Identification of residues critical for metallo-beta-lactamase function by codon randomization and selection
- PMID: 11714924
- PMCID: PMC2374027
- DOI: 10.1110/ps.40884
Identification of residues critical for metallo-beta-lactamase function by codon randomization and selection
Abstract
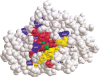
IMP-1 beta-lactamase is a zinc metallo-enzyme encoded by the transferable bla(IMP-1) gene, which confers resistance to virtually all beta-lactam antibiotics including carbapenems. To understand how IMP-1 recognizes and hydrolyzes beta-lactam antibiotics it is important to determine which amino acid residues are critical for catalysis and which residues control substrate specificity. We randomized 27 individual codons in the bla(IMP-1) gene to create libraries that contain all possible amino acid substitutions at residue positions in and near the active site of IMP-1. Mutants from the random libraries were selected for the ability to confer ampicillin resistance to Escherichia coli. Of the positions randomized, >50% do not tolerate amino acid substitutions, suggesting they are essential for IMP-1 function. The remaining positions tolerate amino acid substitutions and may influence the substrate specificity of the enzyme. Interestingly, kinetic studies for one of the functional mutants, Asn233Ala, indicate that an alanine substitution at this position significantly increases catalytic efficiency as compared with the wild-type enzyme.
Figures
References
-
- Amann, E., Brosius, J., and Ptashne, M. 1983. Vectors bearing a hybrid trp–lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene 25 167–178. - PubMed
-
- Ambler, R.P. 1980. The structure of β-lactamases. Philos. Trans. Roy. Soc. Lond. B Biol. Sci. 289 321–331. - PubMed
-
- Bullock, W.O., Fernandez, J.M., and Short, J.M. 1987. XL1-Blue: A high efficiency plasmid transforming recA Escherichia coli strain with β-galactosidase selection. BioTechniques 5 376–379.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

