Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli
- PMID: 11717277
- PMCID: PMC95567
- DOI: 10.1128/JB.183.24.7182-7189.2001
Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli
Abstract
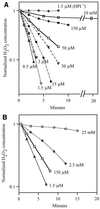
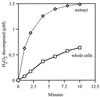
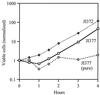
Escherichia coli generates about 14 microM hydrogen peroxide (H(2)O(2)) per s when it grows exponentially in glucose medium. The steady-state intracellular concentration of H(2)O(2) depends on the rates at which this H(2)O(2) is dissipated by scavenging enzymes and by efflux from the cell. The rates of H(2)O(2) degradation by the two major scavenging enzymes, alkyl hydroperoxide reductase and catalase, were quantified. In order to estimate the rate of efflux, the permeability coefficient of membranes for H(2)O(2) was determined. The coefficient is 1.6 x 10(-3) cm/s, indicating that permeability is substantial but not unlimited. These data allowed internal H(2)O(2) fluxes and concentrations to be calculated. Under these growth conditions, Ahp scavenges the majority of the endogenous H(2)O(2), with a small fraction degraded by catalase and virtually none persisting long enough to penetrate the membrane and exit the cell. The robust scavenging activity maintains the H(2)O(2) concentration inside glucose-grown cells at <10(-7) M, substantially below the level (10(-6) M) at which toxicity is evident. When extracellular H(2)O(2) is present, its flux into the cell can be rapid, but the internal concentration may still be an order of magnitude lower than that outside. The presence of such gradients was confirmed in experiments that revealed different degrees of oxidative stress in cocultured scavenger-deficient mutants. The limited permeability of membranes to H(2)O(2) rationalizes the compartmentalization of scavenging systems and predicts that bacteria that excrete redox-cycling drugs do not experience the same H(2)O(2) dose that they impose on their competitors.
Figures

References
-
- Brunder W, Schmidt H, Karch H. KatP, a novel catalase-peroxidase encoded by the large plasmid of enterohaemorrhagic Escherichia coli O157:H7. Microbiology. 1996;142:3305–3315. - PubMed
-
- Fettiplace R. The influence of the lipid on the water permeability of artificial membranes. Biochim Biophys Acta. 1978;513:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

