Redox signal transduction by the ArcB sensor kinase of Haemophilus influenzae lacking the PAS domain
- PMID: 11717280
- PMCID: PMC95570
- DOI: 10.1128/JB.183.24.7206-7212.2001
Redox signal transduction by the ArcB sensor kinase of Haemophilus influenzae lacking the PAS domain
Abstract
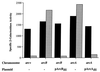
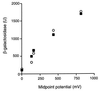
The Arc (anoxic redox control) two-component signal transduction system of Escherichia coli, which comprises the tripartite ArcB sensor kinase and the ArcA response regulator, modulates the expression of numerous operons in response to redox conditions of growth. We demonstrate that the arcA and arcB genes of Haemophilus influenzae specify a two-component system. The Arc proteins of the two bacterial species sufficiently resemble each other that they can participate in heterologous transphosphorylation in vitro. Moreover, the Arc system of H. influenzae mediates transcriptional control according to the redox condition of growth both autologously in its own host and homologously in E. coli, indicating a high degree of functional conservation of the signal transduction system. The H. influenzae ArcB, however, lacks the PAS domain present in the region of E. coli ArcB linking the transmembrane to the cytosolic catalytic domains. Because the PAS domain participates in signal reception in a variety of sensory proteins, including sensors of molecular oxygen and redox state, a similar role was previously ascribed to it in ArcB. Our results demonstrate that the ArcB protein of H. influenzae mediates signal transduction in response to redox conditions of growth despite the absence of the PAS domain.
Figures




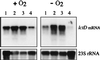
References
-
- Aiba H, Mizuno T, Mizushima S. Transfer of phosphoryl group between two regulatory proteins involved in osmoregulatory expression of the ompF and ompC genes in Escherichia coli. J Biol Chem. 1989;264:8563–8567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

