Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo
- PMID: 11717346
- PMCID: PMC6763925
- DOI: 10.1523/JNEUROSCI.21-23-09134.2001
Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo
Abstract
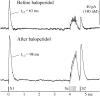
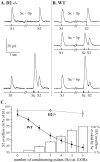
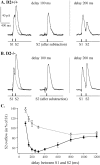
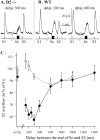
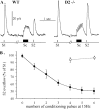
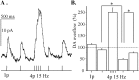
Most neurotransmitters inhibit their own release through autoreceptors. However, the physiological functions of these presynaptic inhibitions are still poorly understood, in part because their time course and functional characteristics have not been described in vivo. Dopamine inhibits its own release through D2 autoreceptors. Here, the part played by autoinhibition in the relationship between impulse flow and dopamine release was studied in vivo in real time. Dopamine release was evoked in the striatum of anesthetized mice by electrical stimulation of the medial forebrain bundle and was continuously monitored by amperometry using carbon fiber electrodes. Control experiments performed in mice lacking D2 receptors showed no autoinhibition of dopamine release. In wild-type mice, stimulation at 100 Hz with two to six pulses linearly inhibited further release, whereas single pulses were inefficient. Dopaminergic neurons exhibit two discharge patterns: single spikes forming a tonic activity below 4 Hz and bursts of two to six action potentials at 15 Hz. Stimulation mimicking one burst (four pulses at 15 Hz) promoted extracellular dopamine accumulation and thus inhibited further dopamine release. This autoinhibition was maximal between 150 and 300 msec after stimulation and disappeared within 600 msec. This delayed and prolonged time course is not reflected in extracellular DA availability and thus probably attributable to mechanisms downstream from autoreceptor stimulation. Thus, in physiological conditions, autoinhibition has two important roles. First, it contributes to the attenuation of extracellular dopamine during bursts. Second, autoinhibition elicited by one burst transiently attenuates further dopamine release elicited by tonic activity.
Figures
References
-
- Agneter E, Hoffmann IS, Singer EA, Cubeddu LX. Behavior of mesocortical dopamine terminals during single and repetitive stimulation: comparison with nigrostriatal neurons. J Pharmacol Exp Ther. 1994;269:470–476. - PubMed
-
- Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J Neurophysiol. 2000;84:1194–1203. - PubMed
-
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. - PubMed
-
- Benoit-Marand M, Jaber M, Gonon F. Release and elimination of dopamine in vivo in mice lacking the dopamine transporter: functional consequences. Eur J Neurosci. 2000;12:2985–2992. - PubMed
-
- Calabresi P, Pisani A, Centonze D, Bernardi G. Synaptic plasticity and physiological interactions between dopamine and glutamate in the striatum. Neurosci Biobehav Rev. 1997;21:519–523. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
