Distance-dependent increase in AMPA receptor number in the dendrites of adult hippocampal CA1 pyramidal neurons
- PMID: 11717348
- PMCID: PMC6763889
- DOI: 10.1523/JNEUROSCI.21-23-09151.2001
Distance-dependent increase in AMPA receptor number in the dendrites of adult hippocampal CA1 pyramidal neurons
Abstract
The Schaffer collateral pathway provides hippocampal CA1 pyramidal cells with a fairly homogeneous excitatory synaptic input that is spread out across several hundred micrometers of their apical dendritic arborizations. A progressive increase in synaptic conductance, with distance from the soma, has been reported to reduce the location dependence that should result from this arrangement. The excitatory synaptic contacts within this pathway primarily use AMPA- and NMDA-type glutamate receptors. To investigate the underlying mechanism of the increased distal excitatory postsynaptic conductance, we used outside-out patches and a fast application system to characterize the properties and distribution of synaptic glutamate receptors across the range of apical dendrites receiving Schaffer collateral input. We observed an approximately twofold increase in AMPA-mediated current amplitude (0.3-0.6 nA) in the range of CA1 apical dendrites that receive a uniform density of Schaffer collateral input (approximately 100-250 micrometer from soma). NMDA-mediated current amplitude, however, remained unchanged. We analyzed the current kinetics, agonist affinity, single-channel conductance, maximum open probability, and reversal potential of AMPA receptors and did not find any differences. Instead, the number of AMPA receptors present in our patches increased approximately twofold. These data suggest that an increase in the number of AMPA receptors present at distal synapses may play an important role in the distance-dependent scaling of Schaffer collateral synapses.
Figures



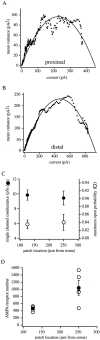



References
-
- Alvarez FJ, Dewey DE, Harrington DA, Fyffe RE. Cell-type specific organization of glycine receptor clusters in the mammalian spinal cord. J Comp Neurol. 1997;379:50–70. - PubMed
-
- Bannister NJ, Larkman AU. Dendritic morphology of CA1 pyramidal neurons from the rat hippocampus. II. Spine distribution. J Comp Neurol. 1995;360:161–171. - PubMed
-
- Benke TA, Luthi A, Isaac JT, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
