The chemokine receptor CCR2 mediates the binding and internalization of monocyte chemoattractant protein-1 along brain microvessels
- PMID: 11717355
- PMCID: PMC6763923
- DOI: 10.1523/JNEUROSCI.21-23-09214.2001
The chemokine receptor CCR2 mediates the binding and internalization of monocyte chemoattractant protein-1 along brain microvessels
Abstract
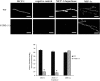
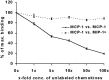
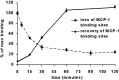
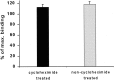
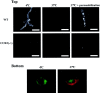
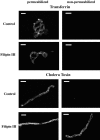
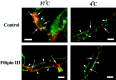
Previous results from this laboratory revealed the presence of high-affinity saturable binding sites for monocyte chemoattractant protein-1 (MCP-1) along human brain microvessels (Andjelkovic et al., 1999; Andjelkovic and Pachter, 2000), which suggested that CC chemokine receptor 2 (CCR2), the recognized receptor for this chemokine, was expressed by the brain microvascular endothelium. To test the role of CCR2 directly in mediating MCP-1 interactions with the brain microvasculature, we assessed MCP-1 binding activity in murine brain microvessels isolated from wild-type mice and from CCR2 (-/-) mice engineered to lack this receptor. Results demonstrate that MCP-1 binding is greatly attenuated in microvessels prepared from CCR2 (-/-) mice compared with wild-type controls. Moreover, microvessels from wild-type mice exhibited MCP-1-induced downmodulation in MCP-1 binding and a recovery of binding activity that was not dependent on de novo protein synthesis. Furthermore, MCP-1 was shown to be internalized within wild-type microvessels, but not within microvessels obtained from CCR2 (-/-) mice, additionally demonstrating that CCR2 is obligatory for MCP-1 endocytosis. Last, internalization of MCP-1, but not transferrin, was observed to be inhibited by disruption of caveolae. Internalized MCP-1 also colocalized at some sites with caveolin-1, a major protein of caveolae, implying that this chemokine is endocytosed, in part, via nonclathrin-coated vesicles. These results prompt consideration that MCP-1 signals may be relayed across the blood-brain barrier by highly specialized interactions of this chemokine with its cognate receptor, CCR2, along brain microvascular endothelial cells.
Figures

References
-
- Abbott NJ, Romero IA. Transporting therapeutics across the blood–brain barrier. Mol Med Today. 1996;2:106–113. - PubMed
-
- Amara A, Gall SL, Schwartz O, Salamero J, Montes M, Loetscher P, Baggliolini M, Virelizier JL, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1α-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. - PMC - PubMed
-
- Anderson RGW. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. - PubMed
-
- Andjelkovic AV, Pachter JS. Characterization of binding sites for chemokines MCP-1 and MIP-1α on human brain microvessels. J Neurochem. 2000;75:1898–1906. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
