The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans
- PMID: 11717360
- PMCID: PMC6763909
- DOI: 10.1523/JNEUROSCI.21-23-09265.2001
The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans
Abstract
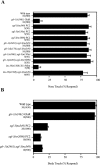
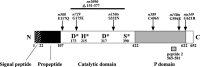
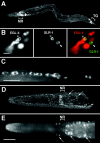
Neuroactive peptides are packaged as proproteins into dense core vesicles or secretory granules, where they are cleaved at dibasic residues by copackaged proprotein convertases. We show here that the Caenorhabditis elegans egl-3 gene encodes a protein that is 57% identical to mouse proprotein convertase type 2 (PC2), and we provide evidence that this convertase regulates mechanosensory responses. Nose touch sensitivity (mediated by ASH sensory neurons) is defective in mutants lacking GLR-1 glutamate receptors (GluRs); however, mutations eliminating the egl-3 PC2 restored nose touch sensitivity to glr-1 GluR mutants. By contrast, body touch sensitivity (mediated by the touch cells) is greatly diminished in egl-3 PC2 mutants. Taken together, these results suggest that egl-3 PC2-processed peptides normally regulate the responsiveness of C. elegans to mechanical stimuli.
Figures

Similar articles
-
The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions.J Neurosci. 2003 Mar 15;23(6):2122-30. doi: 10.1523/JNEUROSCI.23-06-02122.2003. J Neurosci. 2003. PMID: 12657671 Free PMC article.
-
Decreased sensory stimulation reduces behavioral responding, retards development, and alters neuronal connectivity in Caenorhabditis elegans.J Neurosci. 2005 Aug 3;25(31):7159-68. doi: 10.1523/JNEUROSCI.1833-05.2005. J Neurosci. 2005. PMID: 16079398 Free PMC article.
-
Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor.Nature. 1995 Nov 2;378(6552):82-5. doi: 10.1038/378082a0. Nature. 1995. PMID: 7477294
-
Touch sensation in Caenorhabditis elegans.Bioessays. 1996 Mar;18(3):199-206. doi: 10.1002/bies.950180307. Bioessays. 1996. PMID: 8867734 Review.
-
Mechanosensory molecules and circuits in C. elegans.Pflugers Arch. 2015 Jan;467(1):39-48. doi: 10.1007/s00424-014-1574-3. Epub 2014 Jul 23. Pflugers Arch. 2015. PMID: 25053538 Free PMC article. Review.
Cited by
-
Control of Neuropeptide Expression by Parallel Activity-dependent Pathways in Caenorhabditis elegans.Sci Rep. 2017 Jan 31;7:38734. doi: 10.1038/srep38734. Sci Rep. 2017. PMID: 28139692 Free PMC article.
-
Two Rab2 interactors regulate dense-core vesicle maturation.Neuron. 2014 Apr 2;82(1):167-80. doi: 10.1016/j.neuron.2014.02.017. Neuron. 2014. PMID: 24698274 Free PMC article.
-
Neuropeptide-Driven Cross-Modal Plasticity following Sensory Loss in Caenorhabditis elegans.PLoS Biol. 2016 Jan 8;14(1):e1002348. doi: 10.1371/journal.pbio.1002348. eCollection 2016 Jan. PLoS Biol. 2016. Update in: PLoS Biol. 2024 Jul 18;22(7):e3002729. doi: 10.1371/journal.pbio.3002729. PMID: 26745270 Free PMC article. Updated.
-
Molecular profiling of adult C. elegans glia across sexes by single-nuclear RNA-seq.Dev Cell. 2025 Jun 11:S1534-5807(25)00324-7. doi: 10.1016/j.devcel.2025.05.013. Online ahead of print. Dev Cell. 2025. PMID: 40527319
-
Attenuation of insulin signalling contributes to FSN-1-mediated regulation of synapse development.EMBO J. 2013 Jun 12;32(12):1745-60. doi: 10.1038/emboj.2013.91. Epub 2013 May 10. EMBO J. 2013. PMID: 23665919 Free PMC article.
References
-
- Bargmann CI, Thomas JH, Horvitz HR. Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol. 1990;55:529–538. - PubMed
-
- Bellochio E, Reimer R, Fremeau R, Edwards R. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. - PubMed
-
- Besson J-M, Chaouch A. Peripheral and spinal mechanisms of nociception. Phys Rev. 1987;67:67–186. - PubMed
-
- Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
