Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins
- PMID: 11717367
- PMCID: PMC6763918
- DOI: 10.1523/JNEUROSCI.21-23-09334.2001
Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins
Abstract
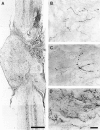
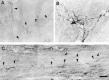
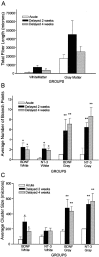
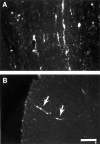
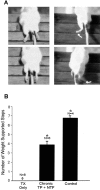
Little axonal regeneration occurs after spinal cord injury in adult mammals. Regrowth of mature CNS axons can be induced, however, by altering the intrinsic capacity of the neurons for growth or by providing a permissive environment at the injury site. Fetal spinal cord transplants and neurotrophins were used to influence axonal regeneration in the adult rat after complete spinal cord transection at a midthoracic level. Transplants were placed into the lesion cavity either immediately after transection (acute injury) or after a 2-4 week delay (delayed or chronic transplants), and either vehicle or neurotrophic factors were administered exogenously via an implanted minipump. Host axons grew into the transplant in all groups. Surprisingly, regeneration from supraspinal pathways and recovery of motor function were dramatically increased when transplants and neurotrophins were delayed until 2-4 weeks after transection rather than applied acutely. Axonal growth back into the spinal cord below the lesion and transplants was seen only in the presence of neurotrophic factors. Furthermore, the restoration of anatomical connections across the injury site was associated with recovery of function with animals exhibiting plantar foot placement and weight-supported stepping. These findings suggest that the opportunity for intervention after spinal cord injury may be greater than originally envisioned and that CNS neurons with long-standing injuries can reinitiate growth, leading to improvement in motor function.
Figures


References
-
- Aguayo A, David S, Richardson P, Bray G. Axonal elongation in peripheral and central nervous system transplants. Adv Cell Neurobiol. 1979;3:215–234.
-
- Aguayo A, David S, Bray G. Influences of the glial environment on the elongation of axons after injury: transplantation studies in adult rodents. J Exp Biol. 1981;95:231–240. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. - PubMed
-
- Bregman B. Development of serotonin immunoreactivity in the rat spinal cord and its plasticity after neonatal spinal cord lesions. Dev Brain Res. 1987a;34:245–263. - PubMed
-
- Bregman B. Spinal cord transplants permit the growth of serotonergic axons across the site of neonatal spinal cord transection. Dev Brain Res. 1987b;34:265–279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
