Lymnaea epidermal growth factor promotes axonal regeneration in CNS organ culture
- PMID: 11717368
- PMCID: PMC6763908
- DOI: 10.1523/JNEUROSCI.21-23-09345.2001
Lymnaea epidermal growth factor promotes axonal regeneration in CNS organ culture
Abstract
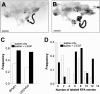
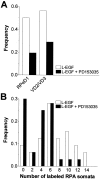
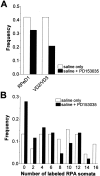
Members of the epidermal growth factor (EGF) family are frequently implicated in the injury response of the mammalian nervous system. Although this implication is supported by extensive molecular evidence, it is not underpinned by conclusive functional data. Recently, we found that expression of an EGF homolog from the pond snail Lymnaea stagnalis (L-EGF) is upregulated after axotomy in the adult CNS, suggesting a role for this molecule in the injury response of the CNS. In the present study we asked whether L-EGF can promote axonal regeneration of three types of identified neurons in organ-cultured CNS. Treatment with purified L-EGF substantially enhanced axonal regeneration of all three types of neurons, an effect inhibited by submicromolar doses of PD153035, a specific EGF receptor (EGFR) tyrosine kinase inhibitor. In addition, PD153035 and K252a, a nonspecific kinase inhibitor, also reduced the degree of axonal regeneration that occurs without L-EGF supplementation, indicating that L-EGF or other EGFR ligands synthesized in the CNS participate in the regenerative response. An intriguing aspect of these results is that axonal regeneration of different, intrinsically L-EGF responsive and unresponsive neurons occurred in a coordinated manner. This observation suggests that indirect in addition to direct actions contribute to the beneficial effect of L-EGF. In conclusion, we provide functional evidence that an EGF homolog can promote axonal regeneration, substantiating existing molecular evidence implicating the EGF family in peripheral nerve regeneration and emphasizes the therapeutic potential of these molecules.
Figures
Similar articles
-
Identification of the role of C/EBP in neurite regeneration following microarray analysis of a L. stagnalis CNS injury model.BMC Neurosci. 2012 Jan 4;13:2. doi: 10.1186/1471-2202-13-2. BMC Neurosci. 2012. PMID: 22217148 Free PMC article.
-
Epidermal growth factor-dependent enhancement of axonal regeneration in the pond snail Lymnaea stagnalis: role of phagocyte survival.J Comp Neurol. 2005 Nov 28;492(4):383-400. doi: 10.1002/cne.20732. J Comp Neurol. 2005. PMID: 16228994
-
Epidermal growth factor receptor inhibitors promote CNS axon growth through off-target effects on glia.Neurobiol Dis. 2009 Oct;36(1):142-50. doi: 10.1016/j.nbd.2009.07.016. Epub 2009 Jul 24. Neurobiol Dis. 2009. PMID: 19632327
-
Canadian Association of Neuroscience review: axonal regeneration in the peripheral and central nervous systems--current issues and advances.Can J Neurol Sci. 2004 May;31(2):142-56. doi: 10.1017/s0317167100053798. Can J Neurol Sci. 2004. PMID: 15198438 Review.
-
The role of neurotrophic factors in nerve regeneration.Neurosurg Focus. 2009 Feb;26(2):E3. doi: 10.3171/FOC.2009.26.2.E3. Neurosurg Focus. 2009. PMID: 19228105 Review.
Cited by
-
APP Binds to the EGFR Ligands HB-EGF and EGF, Acting Synergistically with EGF to Promote ERK Signaling and Neuritogenesis.Mol Neurobiol. 2021 Feb;58(2):668-688. doi: 10.1007/s12035-020-02139-2. Epub 2020 Oct 2. Mol Neurobiol. 2021. PMID: 33009641
-
Spatiotemporal expression profiling of proteins in rat sciatic nerve regeneration using reverse phase protein arrays.Proteome Sci. 2012 Feb 10;10(1):9. doi: 10.1186/1477-5956-10-9. Proteome Sci. 2012. PMID: 22325251 Free PMC article.
-
Identification of the role of C/EBP in neurite regeneration following microarray analysis of a L. stagnalis CNS injury model.BMC Neurosci. 2012 Jan 4;13:2. doi: 10.1186/1471-2202-13-2. BMC Neurosci. 2012. PMID: 22217148 Free PMC article.
-
Identification and localization of growth factor genes in the sea cucumber, Holothuria scabra.Heliyon. 2021 Nov 13;7(11):e08370. doi: 10.1016/j.heliyon.2021.e08370. eCollection 2021 Nov. Heliyon. 2021. PMID: 34825084 Free PMC article.
-
Analysis of FK506-mediated functional recovery and neuroprotection in a rat model of spinal cord injury indicates that EGF is modulated in astrocytes.Exp Ther Med. 2018 Aug;16(2):501-510. doi: 10.3892/etm.2018.6283. Epub 2018 Jun 8. Exp Ther Med. 2018. PMID: 30116308 Free PMC article.
References
-
- Allison P, Benjamin PR. Anatomical studies of central regeneration of an identified molluscan interneuron. Proc R Soc Lond B Biol Sci. 1985;226:135–157.
-
- Ambron RT, Walters ET. Priming events and retrograde injury signals. Mol Neurobiol. 1996;13:61–79. - PubMed
-
- Dubuisson AS, Beuermann RW, Kline DG. Sciatic nerve regeneration across gaps within collagen chambers: the influence of epidermal growth factor. J Reconstr Microsurg. 1993;9:341–346. - PubMed
-
- Ebadi M, Bashir RM, Heidrick ML, Hamada FM, El Refaey H, Hamed A, Helal G, Baxi MD, Cerutis DR, Lassi NK. Neurotrophins and their receptors in nerve injury and repair. Neurochem Int. 1997;30:347–374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
