Functional interactions between tumor and peripheral nerve: changes in excitability and morphology of primary afferent fibers in a murine model of cancer pain
- PMID: 11717370
- PMCID: PMC6763931
- DOI: 10.1523/JNEUROSCI.21-23-09367.2001
Functional interactions between tumor and peripheral nerve: changes in excitability and morphology of primary afferent fibers in a murine model of cancer pain
Abstract
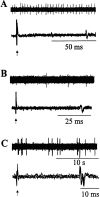
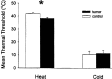
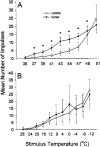
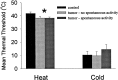
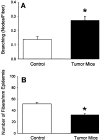
We used a murine model to investigate functional interactions between tumors and peripheral nerves that may contribute to pain associated with cancer. Implantation of fibrosarcoma cells in and around the calcaneus bone produced mechanical hyperalgesia of the ipsilateral paw. Electrophysiological recordings from primary afferent fibers in control and hyperalgesic mice with tumor revealed the development of spontaneous activity (0.2-3.4 Hz) in 34% of cutaneous C-fibers adjacent to the tumor (9-17 d after implantation). C-fibers in tumor-bearing mice exhibited a mean decrease in heat threshold of 3.5 +/- 0.10 degrees C. We also examined innervation of the skin overlying the tumor. Epidermal nerve fibers (ENFs) were immunostained for protein gene product 9.5, imaged using confocal microscopy, and analyzed in terms of number of fibers per millimeter of epidermal length and branching (number of nodes per fiber). Divergent morphological changes were linked to tumor progression. Although branching of ENFs increased significantly relative to control values, in later stages (16-24 d after implantation) of tumor growth a sharp decrease in the number of ENFs was observed. This decay of epidermal innervation of skin over the tumor coincided temporally with gradual loss of electrophysiological activity in tumor-bearing mice. The development of spontaneous activity and sensitization to heat in C-fibers and increased innervation of cutaneous structures within the first 2 weeks of tumor growth suggest activation and sensitization of a proportion of C-fibers. The decrease in the number of ENFs observed in later stages of tumor development implicates neuropathic involvement in this model of cancer pain.
Figures


References
-
- Ali Z, Ringkamp M, Hartke TV, Chien HF, Flavahan NA, Campbell JN, Meyer RA. Uninjured C-fiber nociceptors develop spontaneous activity and alpha-adrenergic sensitivity following L6 spinal nerve ligation in monkey. J Neurophysiol. 1999;81:455–466. - PubMed
-
- Allen BJ, Li J, Menning PM, Rogers SD, Ghilardi J, Mantyh PW, Simone DA. Primary afferent fibers that contribute to increased substance P receptor internalization in the spinal cord after injury. J Neurophysiol. 1999;81:1379–1390. - PubMed
-
- Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol. 1999;82:2649–2656. - PubMed
-
- Baumann TK, Simone DA, Shain CN, Lamotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
