RelE, a global inhibitor of translation, is activated during nutritional stress
- PMID: 11717402
- PMCID: PMC64681
- DOI: 10.1073/pnas.251327898
RelE, a global inhibitor of translation, is activated during nutritional stress
Abstract
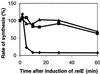
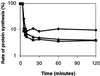
The stringent response is defined as the physiological changes elicited by amino acid starvation. Many of these changes depend on the regulatory nucleotide ppGpp (guanosine tetraphosphate) synthesized by RelA (ppGpp synthetase I), the relA-encoded protein. The second rel locus of Escherichia coli is called relBE and encodes RelE cytotoxin and RelB antitoxin. RelB counteracts the toxic effect of RelE. In addition, RelB is an autorepressor of relBE transcription. Here we reveal a ppGpp-independent mechanism that reduces the level of translation during amino acid starvation. Artificial overexpression of RelE severely inhibited translation. During amino acid starvation, the presence of relBE caused a significant reduction in the poststarvation level of translation. Concomitantly, relBE transcription was rapidly and strongly induced. Induction of transcription occurred independently of relA and spoT (encoding ppGpp synthetase II), but instead depended on Lon protease. Consistently, Lon was required for degradation of RelB. Replacement of the relBE promoter with a LacI-regulated promoter indicated that strong and ongoing transcription of relBE is required to maintain a proper RelB:RelE ratio during starvation. Thus relBE may be regarded as a previously uncharacterized type of stress-response element that reduces the global level of translation during nutritional stress.
Figures



References
-
- Cashel M, Gentry D, Hernandez V J, Vinella D. In: Escherichia coli and Salmonella. Neidthardt F C, editor. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 1458–1496.
-
- Chatterji D, Kumar O A. Curr Opin Microbiol. 2001;4:160–165. - PubMed
-
- Barker M M, Gaal T, Josaitis C A, Gourse R L. J Mol Biol. 2001;305:673–688. - PubMed
-
- Kingston R E, Chamberlin M J. Cell. 1981;27:523–531. - PubMed
-
- Chatterji D, Fujita N, Ishihama A. Genes Cells. 1998;3:279–287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

