Coexisting conformations of fibronectin in cell culture imaged using fluorescence resonance energy transfer
- PMID: 11717404
- PMCID: PMC64704
- DOI: 10.1073/pnas.251422998
Coexisting conformations of fibronectin in cell culture imaged using fluorescence resonance energy transfer
Abstract
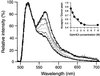
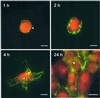
Fluorescence resonance energy transfer (FRET) between fluorophores attached to single proteins provides a tool to study the conformation of proteins in solution and in cell culture. As a protein unfolds, nanometer-scale increases in distance between donor and acceptor fluorophores cause decreases in FRET. Here we demonstrate the application of FRET to imaging coexisting conformations of fibronectin (Fn) in cell culture. Fn is a flexible 440-kDa extracellular matrix protein, with functional sites that are regulated by unfolding events. Fn was labeled with multiple donor and acceptor fluorophores such that intramolecular FRET could be used to distinguish a range of Fn conformations. The sensitivity of FRET to unfolding was tested by progressively denaturing labeled Fn using guanidium chloride. To investigate Fn conformation changes during cell binding and matrix assembly, we added labeled Fn to the culture medium of NIH 3T3 fibroblasts. Coexisting conformations of Fn were visualized using fluorescence microscopy, and spectra from specific features were measured with an attached spectrometer. Using FRET as an indicator of Fn conformation, Fn diffusely bound to cells was in a compact state, whereas Fn in matrix fibrils was highly extended. Matrix fibrils exhibited a range of FRET that suggested some degree of unfolding of Fn's globular modules. Fn in cell-associated clusters that preceded fibril formation appeared more extended than diffuse cell-bound Fn but less extended than fibrillar Fn, suggesting that Fn undergoes extension after cell binding and before polymerization. FRET thus provides an approach to gain insight into the integrin-mediated pathway of Fn fibrillogenesis.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

