A high level of cyclooxygenase-2 inhibitor selectivity is associated with a reduced interference of platelet cyclooxygenase-1 inactivation by aspirin
- PMID: 11717412
- PMCID: PMC64725
- DOI: 10.1073/pnas.251543298
A high level of cyclooxygenase-2 inhibitor selectivity is associated with a reduced interference of platelet cyclooxygenase-1 inactivation by aspirin
Abstract
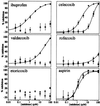
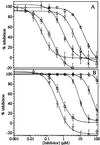
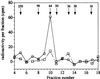
Both nonsteroidal anti-inflammatory drugs, such as ibuprofen, and the prototypical selective cyclooxygenase (Cox)-2 inhibitors DuP-697 and NS-398 block the inhibition of Cox-1 by aspirin in vitro. However, clinical studies have shown that the Cox-2 selective drugs (or coxibs) rofecoxib and etoricoxib, at therapeutic doses, do not interfere with the antiplatelet effect of aspirin, in contrast to ibuprofen. Here, we have evaluated the relative potential of ibuprofen and various coxibs to interfere with the inactivation of Cox-1 by aspirin by using purified enzyme and calcium ionophore-activated human platelets. The irreversible inactivation of Cox-1 by aspirin can be antagonized by ibuprofen and coxibs, albeit with widely different potencies. The rank order of potencies for this process (ibuprofen > celecoxib > valdecoxib > rofecoxib > etoricoxib) parallels that obtained for the inhibition of Cox-1-mediated thromboxane B(2) production by calcium ionophore-stimulated platelets. The antagonism of aspirin therefore likely involves a competition at the enzyme active site. The EC(50) value for the antagonism against 10 microM aspirin for each drug is approximately 10- to 40-fold lower than the corresponding IC(50) value for inhibition of platelet Cox-1 activity, consistent with the much weaker initial binding of aspirin to Cox-1 as compared with arachidonic acid. These results show that a low affinity for Cox-1 and a high degree of Cox-2 selectivity confers a low potential to block aspirin inhibition of platelet Cox-1, consistent with the results of clinical studies.
Figures

References
-
- Marnett L J, Rowlinson S W, Goodwin D C, Kalgutkar A S, Lanzo C A. J Biol Chem. 1999;274:22903–22906. - PubMed
-
- Hawkey C J. Lancet. 1999;353:307–314. - PubMed
-
- Smith W L, DeWitt D L, Garavito R M. Annu Rev Biochem. 2000;69:145–182. - PubMed
-
- Hawkey C J, Jackson L, Harper S E, Simon T J, Mortensen E, Lines C R. Aliment Pharmacol Ther. 2001;15:1–9. - PubMed
-
- Patrono C. Am J Med. 2001;110:S62–S65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

