Peripheral myelin protein 22 is a constituent of intercellular junctions in epithelia
- PMID: 11717414
- PMCID: PMC64694
- DOI: 10.1073/pnas.251548398
Peripheral myelin protein 22 is a constituent of intercellular junctions in epithelia
Abstract
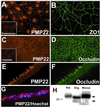
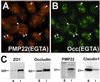
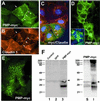
Alterations in peripheral myelin protein 22 (PMP22) gene expression are associated with a host of heritable demyelinating peripheral neuropathies, yet the function of the protein remains unknown. PMP22 expression is highest in myelinating Schwann cells of peripheral nerves; however, significant levels of PMP22 mRNAs can be detected in a variety of non-neural tissue, including epithelia. To date, PMP22 protein expression and localization in non-neural tissues have not been studied in detail. In adult rat liver and intestine, and cultured epithelial cells, we detected PMP22-like immunoreactivity associated with markers of the tight junctional complex, including zonula occludens 1 (ZO-1) and occludin. Upon disruption of intercellular contacts, PMP22 was internalized into vesicles that were immunoreactive for both anti-occludin and anti-PMP22 antibodies. Nonionic detergent extraction of cultured epithelial cells did not solubilize PMP22, as the majority of the protein remained in the detergent insoluble fraction, as did ZO-1 and occludin. We also observed the targeting of exogenous myc-tagged PMP22 to apical cell junctions in polarized epithelia and to anti-ZO-1 antibody immunoreactive cell contacts of L fibroblasts. These studies support a role for PMP22 at intercellular junctions of epithelia and may indicate a similar function in myelinating Schwann cells. Furthermore, our findings could provide an explanation for certain phenotypes of PMP22 neuropathy mice that cannot be accounted for by dysmyelination.
Figures


References
-
- Suter U, Snipes G J. Annu Rev Neurosci. 1995;18:45–75. - PubMed
-
- Taylor V, Welcher A, Suter U. J Biol Chem. 1995;270:28824–28883. - PubMed
-
- Baechner D, Liehr T, Hameister H, Altenberger H, Grehl H. J Neurosci Res. 1995;42:733–741. - PubMed
-
- Wulf P, Bernhardt R, Suter U. J Neurosci Res. 1999;57:467–478. - PubMed
-
- Mugnaini E, Schnapp B. Nature (London) 1974;251:725–727. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

