High refractive index substrates for fluorescence microscopy of biological interfaces with high z contrast
- PMID: 11717428
- PMCID: PMC61094
- DOI: 10.1073/pnas.241208698
High refractive index substrates for fluorescence microscopy of biological interfaces with high z contrast
Abstract
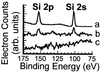
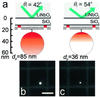
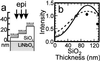
Total internal reflection fluorescence microscopy is widely used to confine the excitation of a complex fluorescent sample very close to the material on which it is supported. By working with high refractive index solid supports, it is possible to confine even further the evanescent field, and by varying the angle of incidence, to obtain quantitative information on the distance of the fluorescent object from the surface. We report the fabrication of hybrid surfaces consisting of nm layers of SiO(2) on lithium niobate (LiNbO(3), n = 2.3). Supported lipid bilayer membranes can be assembled and patterned on these hybrid surfaces as on conventional glass. By varying the angle of incidence of the excitation light, we are able to obtain fluorescent contrast between 40-nm fluorescent beads tethered to a supported bilayer and fluorescently labeled protein printed on the surface, which differ in vertical position by only tens of nm. Preliminary experiments that test theoretical models for the fluorescence-collection factor near a high refractive index surface are presented, and this factor is incorporated into a semiquantitative model used to predict the contrast of the 40-nm bead/protein system. These results demonstrate that it should be possible to profile the vertical location of fluorophores on the nm distance scale in real time, opening the possibility of many experiments at the interface between supported membranes and living cells. Improvements in materials and optical techniques are outlined.
Figures



References
-
- Carrington W A, Lynch R M, Moore E D W, Isenberg G, Fogarty K E, Fay F S. Science. 1995;268:1483–1487. - PubMed
-
- Gustafsson M G L, Agard D A, Sedat J W. J Microsc (Oxford) 1999;195:10–16. - PubMed
-
- Braun D, Fromherz P. Phys Rev Lett. 1998;81:5241–5244.
-
- Bahlmann K, Jakobs S, Hell S W. Ultramicroscopy. 2001;87:155–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

