Mammalian myotube dedifferentiation induced by newt regeneration extract
- PMID: 11717431
- PMCID: PMC61104
- DOI: 10.1073/pnas.221297398
Mammalian myotube dedifferentiation induced by newt regeneration extract
Abstract
Newts are capable of regenerating several anatomical structures and organs, including their limbs. This remarkable regenerative capacity is thought to depend on cellular dedifferentiation. Terminally differentiated mammalian cells, by contrast, are normally incapable of reversing the differentiation process. Several factors could explain the absence of cellular dedifferentiation in mammals: (i) inadequate expression of genes that initiate dedifferentiation; (ii) insufficient intracellular signaling pathways; (iii) irreversible expression of differentiation factors; and (iv) structural characteristics that make dedifferentiation impossible. To investigate the causes underlying the lack of cellular plasticity in mammalian cells, we examined the effect of an extract derived from newt regenerating limbs on terminally differentiated mouse C2C12 myotubes. Approximately 18% of murine myotubes reentered the cell cycle when treated with regeneration extract, whereas 25% of newt myotubes exhibited cell cycle reentry. The muscle differentiation proteins MyoD, myogenin, and troponin T were reduced to undetectable levels in 15-30% of treated murine myotubes. We observed cellular cleavage in 11% of the treated murine myotubes and approximately 50% of these myotubes continued to cleave to produce proliferating mononucleated cells. These data indicate that mammalian myotubes can dedifferentiate when stimulated with the appropriate factors and suggest that one mechanism preventing dedifferentiation of mammalian cells is inadequate spatial or temporal expression of genes that initiate dedifferentiation.
Figures
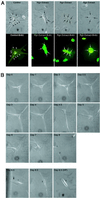


References
-
- Morgan T H. Regeneration. New York: Columbia University; 1901.
-
- Becker R O, Chapin S, Sherry R. Nature (London) 1974;248:145–147. - PubMed
-
- Davis B M, Ayers J L, Koran L, Carlson J, Anderson M C, Simpson S B., Jr Exp Neurol. 1990;108:198–213. - PubMed
-
- Brockes J P. Science. 1997;276:81–87. - PubMed
-
- Zottoli S J, Bentley A P, Feiner D G, Hering J R, Prendergast B J, Rieff H I. Prog Brain Res. 1994;103:219–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

