Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3)
- PMID: 11717447
- PMCID: PMC61138
- DOI: 10.1073/pnas.241516998
Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3)
Abstract
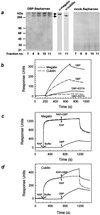
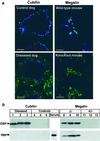
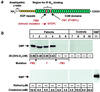
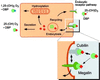
Steroid hormones are central regulators of a variety of biological processes. According to the free hormone hypothesis, steroids enter target cells by passive diffusion. However, recently we demonstrated that 25(OH) vitamin D(3) complexed to its plasma carrier, the vitamin D-binding protein, enters renal proximal tubules by receptor-mediated endocytosis. Knockout mice lacking the endocytic receptor megalin lose 25(OH) vitamin D(3) in the urine and develop bone disease. Here, we report that cubilin, a membrane-associated protein colocalizing with megalin, facilitates the endocytic process by sequestering steroid-carrier complexes on the cellular surface before megalin-mediated internalization of the cubilin-bound ligand. Dogs with an inherited disorder affecting cubilin biosynthesis exhibit abnormal vitamin D metabolism. Similarly, human patients with mutations causing cubilin dysfunction exhibit urinary excretion of 25(OH) vitamin D(3). This observation identifies spontaneous mutations in an endocytic receptor pathway affecting cellular uptake and metabolism of a steroid hormone.
Figures
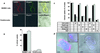
References
-
- Mendel C M. Endocr Rev. 1989;10:232–274. - PubMed
-
- Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Christensen E I, Willnow T. Cell. 1999;96:507–515. - PubMed
-
- Gliemann J. Biol Chem. 1998;379:951–964. - PubMed
-
- Kristiansen M, Kozyraki R, Jacobsen C, Nexo E, Verroust P J, Moestrup S K. J Biol Chem. 1999;274:20540–20544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

