Adeno-associated virus vector-mediated IL-10 gene delivery prevents type 1 diabetes in NOD mice
- PMID: 11717448
- PMCID: PMC61141
- DOI: 10.1073/pnas.251532298
Adeno-associated virus vector-mediated IL-10 gene delivery prevents type 1 diabetes in NOD mice
Abstract
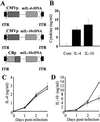
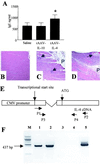
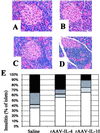
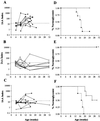
The development of spontaneous autoimmune diabetes in nonobese diabetic (NOD) mice provides for their use as a model of human type 1 diabetes. To test the feasibility of muscle-directed gene therapy to prevent type 1 diabetes, we developed recombinant adeno-associated virus (rAAV) vectors containing murine cDNAs for immunomodulatory cytokines IL-4 or IL-10. Skeletal muscle transduction of female NOD mice with IL-10, but not IL-4, completely abrogated diabetes. rAAV-IL-10 transduction attenuated the production of insulin autoantibodies, quantitatively reduced pancreatic insulitis, maintained islet insulin content, and altered splenocyte cytokine responses to mitogenic stimulation. The beneficial effects were host specific, as adoptive transfer of splenocytes from rAAV IL-10-treated animals rapidly imparted diabetes in naive hosts, and the cells contained no protective immunomodulatory capacity, as defined through adoptive cotransfer analyses. These results indicate the utility for rAAV, a vector with advantages for therapeutic gene delivery, to transfer immunoregulatory cytokines capable of preventing type 1 diabetes. In addition, these studies provide foundational support for the concept of using immunoregulatory agents delivered by rAAV to modulate a variety of disorders associated with deleterious immune responses, including allergic reactions, transplantation rejection, immunodeficiencies, and autoimmune disorders.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

