Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo
- PMID: 11717453
- PMCID: PMC61149
- DOI: 10.1073/pnas.251534698
Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo
Abstract
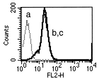
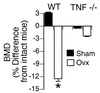
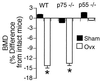
In vivo studies have shown T cells to be central to the mechanism by which estrogen deficiency induces bone loss, but the mechanism involved remains, in part, undefined. In vitro, T cells from ovariectomized mice produce increased amounts of tumor necrosis factor (TNF), which augments receptor activator of NF-kappa B ligand (RANKL)-induced osteoclastogenesis. However, both the mechanism and the relevance of this phenomenon in vivo remain to be established. In this study, we found that ovariectomy increased the number of bone marrow T cell-producing TNF without altering production of TNF per T cell. Attesting to the essential contribution of TNF, ovariectomy induced rapid bone loss in wild type (wt) mice but failed to do so in TNF-deficient (TNF(-/-)) mice. Furthermore, ovariectomy induced bone loss, which was absent in T cell-deficient nude mice, was restored by adoptive transfer of wt T cells, but not by reconstitution with T cells from TNF(-/-) mice. These findings demonstrate the key causal role of T cell-produced TNF in the bone loss after estrogen withdrawal. Finally, ovariectomy caused bone loss in wt mice and in mice lacking p75 TNF receptor but failed to do so in mice lacking the p55 TNF receptor. These findings demonstrate that enhanced T cell production of TNF resulting from increased bone marrow T cell number is a key mechanism by which estrogen deficiency induces bone loss in vivo. The data also demonstrate that the bone-wasting effect of TNF in vivo is mediated by the p55 TNF receptor.
Figures

References
-
- Manolagas S C, Jilka R L. N Engl J Med. 1995;332:305–311. - PubMed
-
- Lacey D L, Timms E, Tan H L, Kelley M J, Dunstan C R, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Cell. 1998;93:165–176. - PubMed
-
- Kong Y Y, Yoshida H, Sarosi I, Tan H L, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos A J, Van G, Itie A, et al. Nature (London) 1999;397:315–323. - PubMed
-
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie M T, Martin T J. Endocr Rev. 1999;20:345–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

