The dissociation of acetylcholine from open nicotinic receptor channels
- PMID: 11717464
- PMCID: PMC61175
- DOI: 10.1073/pnas.251402498
The dissociation of acetylcholine from open nicotinic receptor channels
Erratum in
- Proc Natl Acad Sci U S A 2002 Jan 8;99(1):541
Abstract
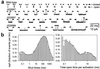
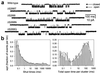
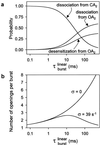
Ligand-gated ion channels bind agonists with higher affinity in the open than in the closed state. The kinetic basis of this increased affinity has remained unknown, because even though the rate constants of agonist association to and dissociation from closed receptors can be estimated with reasonable certainty, the kinetics of the binding steps in open receptors have proven to be elusive. To be able to measure the agonist-dissociation rate constant from open muscle nicotinic receptors, we increased the probability of ligand unbinding from the open state by engineering a number of mutations that speed up opening and slow down closing but leave the ligand-binding properties unchanged. Single-channel patch-clamp recordings from the wild-type and mutant constructs were performed at very low concentrations of acetylcholine (ACh). The durations of individual channel activations were analyzed assuming that "bursts" of fully liganded (diliganded) receptor openings can be terminated by ligand dissociation from the closed or open state (followed by fast closure) or by desensitization. This analysis revealed that ACh dissociates from diliganded open receptors at approximately 24 s(-1), that is, approximately 2,500 times more slowly than from diliganded closed receptors. This change alone without a concomitant change in the association rate constant to the open state quantitatively accounts for the increased equilibrium affinity of the open channel for ACh. Also, the results predict that both desensitization and ACh dissociation from the open state frequently terminate bursts of openings in naturally occurring gain-of-function mutants (which cause slow-channel congenital myasthenia) and therefore would contribute significantly to the time course of the endplate current decay in these disease conditions.
Figures


References
-
- Monod J, Wyman J, Changeux J P. J Mol Biol. 1965;12:88–118. - PubMed
-
- Karlin A. J Theor Biol. 1967;16:306–320. - PubMed
-
- Jackson M B. In: The Nicotinic Acetylcholine Receptor: Current Views and Future Trends. Barrantes F, editor. Austin,TX: Landes Bioscience; 1997. pp. 61–84.
-
- Edelstein S J, Changeux J P. Adv Protein Chem. 1998;51:121–184. - PubMed
-
- Reitstetter R, Lukas R J, Gruener R J. J Pharmacol Exp Ther. 1999;289:656–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

