The DELLA motif is essential for gibberellin-induced degradation of RGA
- PMID: 11717468
- PMCID: PMC61185
- DOI: 10.1073/pnas.251534098
The DELLA motif is essential for gibberellin-induced degradation of RGA
Abstract
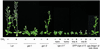
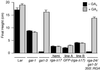
RGA and GAI are homologous genes that encode putative transcriptional regulators that repress gibberellin (GA) signaling in Arabidopsis. Previously we showed that the green fluorescent protein (GFP)-RGA fusion protein is localized to the nucleus in transgenic Arabidopsis, and expression of this fusion protein rescues the rga null mutation. The GA signal seems to derepress the GA response pathway by degrading the repressor protein RGA. The GA-insensitive, semidominant, semidwarf gai-1 mutant encodes a mutant protein with a 17-amino acid deletion within the DELLA domain of GAI. It was hypothesized that this mutation turns the gai protein into a constitutive repressor of GA signaling. Because the sequences missing in gai-1 are identical between GAI and RGA, we tested whether an identical mutation (rga-Delta 17) in the RGA gene would confer a phenotype similar to gai-1. We demonstrated that expression of rga-Delta 17 or GFP-(rga-Delta 17) under the control of the RGA promoter caused a GA-unresponsive severe dwarf phenotype in transgenic Arabidopsis. Analysis of the mRNA levels of a GA biosynthetic gene, GA4, showed that the feedback control of GA biosynthesis in these transgenic plants was less responsive to GA than that in wild type. Immunoblot and confocal microscopy analyses indicated that rga-Delta17 and GFP-(rga-Delta 17) proteins were resistant to degradation after GA application. Our results illustrate that the DELLA domain in RGA plays a regulatory role in GA-induced degradation of RGA. Deletion of this region stabilizes the rga-Delta 17 mutant protein, and regardless of the endogenous GA status rga-Delta 17 becomes a constitutively active repressor of GA signaling.
Figures




Similar articles
-
Arabidopsis RGL1 encodes a negative regulator of gibberellin responses.Plant Cell. 2002 Jan;14(1):87-100. doi: 10.1105/tpc.010325. Plant Cell. 2002. PMID: 11826301 Free PMC article.
-
Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis.Plant Cell. 2001 Jul;13(7):1555-66. doi: 10.1105/tpc.010047. Plant Cell. 2001. PMID: 11449051 Free PMC article.
-
Evidence that the Arabidopsis nuclear gibberellin signalling protein GAI is not destabilised by gibberellin.Plant J. 2002 Dec;32(6):935-47. doi: 10.1046/j.1365-313x.2002.01478.x. Plant J. 2002. PMID: 12492836
-
Molecular biology of gibberellins signaling in higher plants.Int Rev Cell Mol Biol. 2008;268:191-221. doi: 10.1016/S1937-6448(08)00806-X. Int Rev Cell Mol Biol. 2008. PMID: 18703407 Review.
-
DELLA-dependent and -independent gibberellin signaling.Plant Signal Behav. 2018 Mar 4;13(3):e1445933. doi: 10.1080/15592324.2018.1445933. Epub 2018 Mar 22. Plant Signal Behav. 2018. PMID: 29485381 Free PMC article. Review.
Cited by
-
Gibberellin-mediated RGA-LIKE1 degradation regulates embryo sac development in Arabidopsis.J Exp Bot. 2020 Dec 31;71(22):7059-7072. doi: 10.1093/jxb/eraa395. J Exp Bot. 2020. PMID: 32845309 Free PMC article.
-
The Arabidopsis MYB96 Transcription Factor Is a Positive Regulator of ABSCISIC ACID-INSENSITIVE4 in the Control of Seed Germination.Plant Physiol. 2015 Jun;168(2):677-89. doi: 10.1104/pp.15.00162. Epub 2015 Apr 13. Plant Physiol. 2015. PMID: 25869652 Free PMC article.
-
Molecular cloning, expression analysis and subcellular localization of four DELLA genes from hybrid poplar.Springerplus. 2016 Jul 19;5(1):1129. doi: 10.1186/s40064-016-2728-x. eCollection 2016. Springerplus. 2016. PMID: 27478746 Free PMC article.
-
Knockdown of a JmjC domain-containing gene JMJ524 confers altered gibberellin responses by transcriptional regulation of GRAS protein lacking the DELLA domain genes in tomato.J Exp Bot. 2015 Mar;66(5):1413-26. doi: 10.1093/jxb/eru493. Epub 2015 Feb 13. J Exp Bot. 2015. PMID: 25680796 Free PMC article.
-
The DELLA-CONSTANS Transcription Factor Cascade Integrates Gibberellic Acid and Photoperiod Signaling to Regulate Flowering.Plant Physiol. 2016 Sep;172(1):479-88. doi: 10.1104/pp.16.00891. Epub 2016 Jul 12. Plant Physiol. 2016. PMID: 27406167 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

