Interleukin-12 stimulation of lymphoproliferative responses in Trypanosoma cruzi infection
- PMID: 11722650
- PMCID: PMC1783308
- DOI: 10.1046/j.1365-2567.2001.01311.x
Interleukin-12 stimulation of lymphoproliferative responses in Trypanosoma cruzi infection
Abstract
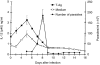
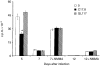
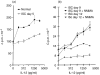
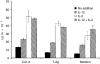
The cytokine interleukin-12 (IL-12) is essential for resistance to Trypanosoma cruzi infection because it stimulates the synthesis of interferon-gamma (IFN-gamma), a major activator of the parasiticidal effect of macrophages. A less studied property of IL-12 is its ability to amplify the proliferation of T or natural killer (NK) lymphocytes. We investigated the role of endogenously produced IL-12 in the maintenance of parasite antigen (T-Ag)-specific lymphoproliferative responses during the acute phase of T. cruzi infection. We also studied whether treatment with recombinant IL-12 (rIL-12) would stimulate T-Ag-specific or concanavalin A (Con A)-stimulated lymphoproliferation and abrogate the suppression that is characteristic of the acute phase of infection. Production of IL-12 by spleen-cell cultures during T. cruzi infection increased in the first days of infection (days 3-5) and decreased as infection progressed beyond day 7. The growth-promoting activity of endogenous IL-12 on T-Ag-specific proliferation was observed on day 5 of infection. Treatment of cultures with rIL-12 promoted a significant increase in Con A-stimulated proliferation by spleen cells from normal or infected mice. Enhanced T-Ag-specific proliferation by rIL-12 was seen in spleen cell cultures from infected mice providing that nitric oxide production was inhibited by treatment with the competitive inhibitor NG-monomethyl-L-arginine (NMMA). Enhancement of proliferation promoted by IL-12 occurred in the presence of neutralizing anti-interleukin-2 (IL-2) antibody, suggesting that this activity of IL-12 was partly independent of endogenous IL-2. Thymidine incorporation levels achieved with rIL-12 treatment of the cultures were approximately 50% of those stimulated by rIL-2 in the same cultures.
Figures


References
-
- Curotto-de-Lafaille MA, Barbosa-De-Oliveira LC, Lima GC, Abrahamsohn IA. Trypanosoma cruzi: maintenance of parasite-specific T cell responses in lymph nodes during the acute phase of infection. Exp Parasitol. 1990;70:164–74. - PubMed
-
- Ramos C, Lamoyi E, Feoli M, Rodriguez M, Perez M, Ortiz-Ortiz L. Trypanosoma cruzi: immunosuppressed response to different antigens induced in the infected mouse. Exp Parasitol. 1978;45:190–9. - PubMed
-
- Abrahamsohn IA, Coffman RL. Cytokine and nitric oxide regulation of the immunosuppression in Trypanosoma cruzi infection. J Immunol. 1995;155:3955–63. - PubMed
-
- Harel-Bellan A, Joskowicz M, Fradelizi D, Eisen H. T lymphocyte function during experimental Chagas' disease: production of and response to interleukin 2. Eur J Immunol. 1985;15:438–42. - PubMed
-
- Pinge-Filho P, Tadokoro CE, Abrahamsohn IA. Prostaglandins mediate suppression of lymphocyte proliferation and cytokine synthesis in acute Trypanosoma cruzi infection. Cell Immunol. 1999;193:90–8. 10.1006/cimm.1999.1463. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

