Identification of a novel metabolite in the degradation of pyrene by Mycobacterium sp. strain AP1: actions of the isolate on two- and three-ring polycyclic aromatic hydrocarbons
- PMID: 11722898
- PMCID: PMC93335
- DOI: 10.1128/AEM.67.12.5497-5505.2001
Identification of a novel metabolite in the degradation of pyrene by Mycobacterium sp. strain AP1: actions of the isolate on two- and three-ring polycyclic aromatic hydrocarbons
Abstract
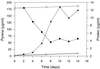
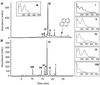
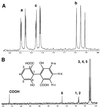
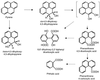
Mycobacterium sp. strain AP1 grew with pyrene as a sole source of carbon and energy. The identification of metabolites accumulating during growth suggests that this strain initiates its attack on pyrene by either monooxygenation or dioxygenation at its C-4, C-5 positions to give trans- or cis-4,5-dihydroxy-4,5-dihydropyrene, respectively. Dehydrogenation of the latter, ortho cleavage of the resulting diol to form phenanthrene 4,5-dicarboxylic acid, and subsequent decarboxylation to phenanthrene 4-carboxylic acid lead to degradation of the phenanthrene 4-carboxylic acid via phthalate. A novel metabolite identified as 6,6'-dihydroxy-2,2'-biphenyl dicarboxylic acid demonstrates a new branch in the pathway that involves the cleavage of both central rings of pyrene. In addition to pyrene, strain AP1 utilized hexadecane, phenanthrene, and fluoranthene for growth. Pyrene-grown cells oxidized the methylenic groups of fluorene and acenaphthene and catalyzed the dihydroxylation and ortho cleavage of one of the rings of naphthalene and phenanthrene to give 2-carboxycinnamic and diphenic acids, respectively. The catabolic versatility of strain AP1 and its use of ortho cleavage mechanisms during the degradation of polycyclic aromatic hydrocarbons (PAHs) give new insight into the role that pyrene-degrading bacterial strains may play in the environmental fate of PAH mixtures.
Figures
Similar articles
-
Metabolism of fluoranthene by mycobacterial strains isolated by their ability to grow in fluoranthene or pyrene.J Ind Microbiol Biotechnol. 2005 Oct;32(10):455-64. doi: 10.1007/s10295-005-0022-y. Epub 2005 Oct 15. J Ind Microbiol Biotechnol. 2005. PMID: 16133098
-
Simultaneous biodegradation of creosote-polycyclic aromatic hydrocarbons by a pyrene-degrading Mycobacterium.Appl Microbiol Biotechnol. 2008 Feb;78(1):165-72. doi: 10.1007/s00253-007-1284-2. Epub 2007 Dec 12. Appl Microbiol Biotechnol. 2008. PMID: 18074131
-
Metabolism of fluoranthene by Mycobacterium sp. strain AP1.Appl Microbiol Biotechnol. 2006 May;70(6):747-56. doi: 10.1007/s00253-005-0120-9. Epub 2005 Aug 25. Appl Microbiol Biotechnol. 2006. PMID: 16133330
-
Bacterial degradation of aromatic compounds.Int J Environ Res Public Health. 2009 Jan;6(1):278-309. doi: 10.3390/ijerph6010278. Epub 2009 Jan 13. Int J Environ Res Public Health. 2009. PMID: 19440284 Free PMC article. Review.
-
Aerobic and Anaerobic Bacterial and Fungal Degradation of Pyrene: Mechanism Pathway Including Biochemical Reaction and Catabolic Genes.Int J Mol Sci. 2021 Jul 30;22(15):8202. doi: 10.3390/ijms22158202. Int J Mol Sci. 2021. PMID: 34360967 Free PMC article. Review.
Cited by
-
Effects of Polycyclic Aromatic Hydrocarbon Mixtures on Degradation, Gene Expression, and Metabolite Production in Four Mycobacterium Species.Appl Environ Microbiol. 2016 May 16;82(11):3357-3369. doi: 10.1128/AEM.00100-16. Print 2016 Jun 1. Appl Environ Microbiol. 2016. PMID: 27037123 Free PMC article.
-
Isolation and characterization of different bacterial strains for bioremediation of n-alkanes and polycyclic aromatic hydrocarbons.Environ Sci Pollut Res Int. 2015 Oct;22(20):15332-46. doi: 10.1007/s11356-015-4343-8. Epub 2015 Mar 28. Environ Sci Pollut Res Int. 2015. PMID: 25813636
-
Metabolism of fluoranthene by mycobacterial strains isolated by their ability to grow in fluoranthene or pyrene.J Ind Microbiol Biotechnol. 2005 Oct;32(10):455-64. doi: 10.1007/s10295-005-0022-y. Epub 2005 Oct 15. J Ind Microbiol Biotechnol. 2005. PMID: 16133098
-
Study of biochemical pathways and enzymes involved in pyrene degradation by Mycobacterium sp. strain KMS.Appl Environ Microbiol. 2006 Dec;72(12):7821-8. doi: 10.1128/AEM.01274-06. Epub 2006 Oct 13. Appl Environ Microbiol. 2006. PMID: 17041157 Free PMC article.
-
Simultaneous Biodegradation of Polyaromatic Hydrocarbons by a Stenotrophomonas sp: Characterization of nid Genes and Effect of Surfactants on Degradation.Indian J Microbiol. 2017 Mar;57(1):60-67. doi: 10.1007/s12088-016-0612-6. Epub 2016 Jul 28. Indian J Microbiol. 2017. PMID: 28148980 Free PMC article.
References
-
- Black T H. The preparation and reactions of diazomethane. Aldrichim Acta. 1983;16:39.
-
- Cerniglia C E. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation. 1992;3:351–368.
-
- Cerniglia C E, Heitkamp M A. Polycyclic aromatic hydrocarbon degradation by Mycobacterium. Methods Enzymol. 1990;188:148–153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

