Benzoate fermentation by the anaerobic bacterium Syntrophus aciditrophicus in the absence of hydrogen-using microorganisms
- PMID: 11722901
- PMCID: PMC93338
- DOI: 10.1128/AEM.67.12.5520-5525.2001
Benzoate fermentation by the anaerobic bacterium Syntrophus aciditrophicus in the absence of hydrogen-using microorganisms
Abstract
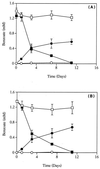
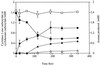
The anaerobic bacterium Syntrophus aciditrophicus metabolized benzoate in pure culture in the absence of hydrogen-utilizing partners or terminal electron acceptors. The pure culture of S. aciditrophicus produced approximately 0.5 mol of cyclohexane carboxylate and 1.5 mol of acetate per mol of benzoate, while a coculture of S. aciditrophicus with the hydrogen-using methanogen Methanospirillum hungatei produced 3 mol of acetate and 0.75 mol of methane per mol of benzoate. The growth yield of the S. aciditrophicus pure culture was 6.9 g (dry weight) per mol of benzoate metabolized, whereas the growth yield of the S. aciditrophicus-M. hungatei coculture was 11.8 g (dry weight) per mol of benzoate. Cyclohexane carboxylate was metabolized by S. aciditrophicus only in a coculture with a hydrogen user and was not metabolized by S. aciditrophicus pure cultures. Cyclohex-1-ene carboxylate was incompletely degraded by S. aciditrophicus pure cultures until a free energy change (DeltaG') of -9.2 kJ/mol was reached (-4.7 kJ/mol for the hydrogen-producing reaction). Cyclohex-1-ene carboxylate, pimelate, and glutarate transiently accumulated at micromolar levels during growth of an S. aciditrophicus pure culture with benzoate. High hydrogen (10.1 kPa) and acetate (60 mM) levels inhibited benzoate metabolism by S. aciditrophicus pure cultures. These results suggest that benzoate fermentation by S. aciditrophicus in the absence of hydrogen users proceeds via a dismutation reaction in which the reducing equivalents produced during oxidation of one benzoate molecule to acetate and carbon dioxide are used to reduce another benzoate molecule to cyclohexane carboxylate, which is not metabolized further. Benzoate fermentation to acetate, CO(2), and cyclohexane carboxylate is thermodynamically favorable and can proceed at free energy values more positive than -20 kJ/mol, the postulated minimum free energy value for substrate metabolism.
Figures
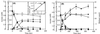
Similar articles
-
Cyclohexane carboxylate and benzoate formation from crotonate in Syntrophus aciditrophicus.Appl Environ Microbiol. 2007 Feb;73(3):930-8. doi: 10.1128/AEM.02227-06. Epub 2006 Dec 8. Appl Environ Microbiol. 2007. PMID: 17158621 Free PMC article.
-
Metabolism of benzoate, cyclohex-1-ene carboxylate, and cyclohexane carboxylate by "Syntrophus aciditrophicus" strain SB in syntrophic association with H(2)-using microorganisms.Appl Environ Microbiol. 2001 Apr;67(4):1728-38. doi: 10.1128/AEM.67.4.1728-1738.2001. Appl Environ Microbiol. 2001. PMID: 11282627 Free PMC article.
-
Use of benzoate as an electron acceptor by Syntrophus aciditrophicus grown in pure culture with crotonate.Environ Microbiol. 2008 Dec;10(12):3265-74. doi: 10.1111/j.1462-2920.2008.01716.x. Epub 2008 Aug 13. Environ Microbiol. 2008. PMID: 18707608
-
Fermentative Cyclohexane Carboxylate Formation in Syntrophus aciditrophicus.J Mol Microbiol Biotechnol. 2016;26(1-3):165-79. doi: 10.1159/000440881. Epub 2016 Mar 10. J Mol Microbiol Biotechnol. 2016. PMID: 26959729 Review.
-
Metabolic interactions between anaerobic bacteria in methanogenic environments.Antonie Van Leeuwenhoek. 1994;66(1-3):271-94. doi: 10.1007/BF00871644. Antonie Van Leeuwenhoek. 1994. PMID: 7747937 Review.
Cited by
-
The role of syntrophic associations in sustaining anaerobic mineralization of chlorinated organic compounds.Environ Health Perspect. 2005 Mar;113(3):310-6. doi: 10.1289/ehp.6933. Environ Health Perspect. 2005. PMID: 15743720 Free PMC article.
-
Anaerobic catabolism of aromatic compounds: a genetic and genomic view.Microbiol Mol Biol Rev. 2009 Mar;73(1):71-133. doi: 10.1128/MMBR.00021-08. Microbiol Mol Biol Rev. 2009. PMID: 19258534 Free PMC article. Review.
-
Cyclohexane carboxylate and benzoate formation from crotonate in Syntrophus aciditrophicus.Appl Environ Microbiol. 2007 Feb;73(3):930-8. doi: 10.1128/AEM.02227-06. Epub 2006 Dec 8. Appl Environ Microbiol. 2007. PMID: 17158621 Free PMC article.
-
Impact of Silver and Copper Oxide Nanoparticles on Anaerobic Digestion of Sludge and Bacterial Community Structure.Nanomaterials (Basel). 2025 Feb 3;15(3):236. doi: 10.3390/nano15030236. Nanomaterials (Basel). 2025. PMID: 39940212 Free PMC article.
-
Cyclohexanecarboxyl-coenzyme A (CoA) and cyclohex-1-ene-1-carboxyl-CoA dehydrogenases, two enzymes involved in the fermentation of benzoate and crotonate in Syntrophus aciditrophicus.J Bacteriol. 2013 Jul;195(14):3193-200. doi: 10.1128/JB.00322-13. Epub 2013 May 10. J Bacteriol. 2013. PMID: 23667239 Free PMC article.
References
-
- Amos D A, McInerney M J. Growth of Syntrophomonas wolfei on unsaturated short chain fatty acids. Arch Microbiol. 1990;154:31–36.
-
- Auburger G, Winter J. Isolation and physiological characterization of Syntrophus buswellii strain GA from a syntrophic benzoate-degrading, strictly anaerobic coculture. Appl Microbiol Biotechnol. 1995;44:241–248.
-
- Barker H A. On the fermentation of some dibasic C4 acids by Aerobacter aerogenes. K Akad Wetenschappen Amsterdam Proc. 1936;39:674–683.
-
- Beaty P S, McInerney M J. Growth of Syntrophomonas wolfei in pure culture on crotonate. Arch Microbiol. 1987;147:389–393.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

