Growth and detachment of cell clusters from mature mixed-species biofilms
- PMID: 11722913
- PMCID: PMC93350
- DOI: 10.1128/AEM.67.12.5608-5613.2001
Growth and detachment of cell clusters from mature mixed-species biofilms
Abstract
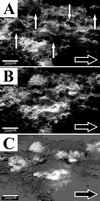
Detachment from biofilms is an important consideration in the dissemination of infection and the contamination of industrial systems but is the least-studied biofilm process. By using digital time-lapse microscopy and biofilm flow cells, we visualized localized growth and detachment of discrete cell clusters in mature mixed-species biofilms growing under steady conditions in turbulent flow in situ. The detaching biomass ranged from single cells to an aggregate with a diameter of approximately 500 microm. Direct evidence of local cell cluster detachment from the biofilms was supported by microscopic examination of filtered effluent. Single cells and small clusters detached more frequently, but larger aggregates contained a disproportionately high fraction of total detached biomass. These results have significance in the establishment of an infectious dose and public health risk assessment.
Figures


References
-
- Bakke R, Olsson P Q. Biofilm thickness measurements by light-microscopy. J Microbiol Methods. 1986;5:93–98.
-
- Bryers J D. Modeling biofilm accumulation. In: Bazin M J, Prosser J I, editors. Physiological models in microbiology. Vol. 2. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 109–144.
-
- Characklis W G. Biofilm processes. In: Characklis W G, Marshall K C, editors. Biofilms. New York, N.Y: John Wiley & Sons, Inc.; 1990. pp. 195–231.
-
- Dalton H M, Goodman A E, Marshall K C. Diversity in surface colonization behavior in marine bacteria. J Ind Microbiol. 1996;17:228–234.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

