Green fluorescent protein as a novel indicator of antimicrobial susceptibility in Aureobasidium pullulans
- PMID: 11722914
- PMCID: PMC93351
- DOI: 10.1128/AEM.67.12.5614-5620.2001
Green fluorescent protein as a novel indicator of antimicrobial susceptibility in Aureobasidium pullulans
Abstract
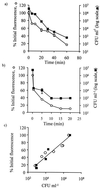
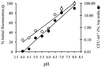
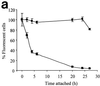
Presently there is no method available that allows noninvasive and real-time monitoring of fungal susceptibility to antimicrobial compounds. The green fluorescent protein (GFP) of the jellyfish Aequoria victoria was tested as a potential reporter molecule for this purpose. Aureobasidium pullulans was transformed to express cytosolic GFP using the vector pTEFEGFP (A. J. Vanden Wymelenberg, D. Cullen, R. N. Spear, B. Schoenike, and J. H. Andrews, BioTechniques 23:686-690, 1997). The transformed strain Ap1 gfp showed bright fluorescence that was amenable to quantification using fluorescence spectrophotometry. Fluorescence levels in Ap1 gfp blastospore suspensions were directly proportional to the number of viable cells determined by CFU plate counts (r(2) > 0.99). The relationship between cell viability and GFP fluorescence was investigated by adding a range of concentrations of each of the biocides sodium hypochlorite and 2-n-octylisothiozolin-3-one (OIT) to suspensions of Ap1 gfp blastospores (pH 5 buffer). These biocides each caused a rapid (< 25-min) loss of fluorescence of greater than 90% when used at concentrations of 150 microg of available chlorine ml(-1) and 500 microg ml(-1), respectively. Further, loss of GFP fluorescence from A. pullulans cells was highly correlated with a decrease in the number of viable cells (r(2) > 0.92). Losses of GFP fluorescence and cell viability were highly dependent on external pH; maximum losses of fluorescence and viability occurred at pH 4, while reduction of GFP fluorescence was absent at pH 8.0 and was associated with a lower reduction in viability. When A. pullulans was attached to the surface of plasticized poly(vinylchloride) containing 500 ppm of OIT, fluorescence decreased more slowly than in cell suspensions, with > 95% loss of fluorescence after 27 h. This technique should have broad applications in testing the susceptibility of A. pullulans and other fungal species to antimicrobial compounds.
Figures



References
-
- Baldwin B C, Wiggins T E. Action of fungicidal triazoles of the dichlorobutrazol series on Ustilago maydis. Pesticide Sci. 1984;15:156–166.
-
- Ben-Josef A M, Manavathu E K, Platt D, Sobel J D. Proton translocating ATPase mediated fungicidal activity of a novel complex carbohydrate: CAN-296. Int J Antimicrob Agents. 2000;13:287–295. - PubMed
-
- Casey W M, Nguyen N T. Use of the green fluorescent protein to rapidly assess viability of Escherichia coli in preserved solutions. PDA J Pharm Sci Technol. 1995;50:352–355. - PubMed
-
- Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. - PubMed
-
- Cheng L, Fu J, Tsukamoto A, Hawley R G. Use of green fluorescent protein variants to monitor gene transfer and expression in mammalian cells. Nat Biotechnol. 1996;14:606–609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

