The alpha-helix 4 residue, Asn135, is involved in the oligomerization of Cry1Ac1 and Cry1Ab5 Bacillus thuringiensis toxins
- PMID: 11722927
- PMCID: PMC93364
- DOI: 10.1128/AEM.67.12.5715-5720.2001
The alpha-helix 4 residue, Asn135, is involved in the oligomerization of Cry1Ac1 and Cry1Ab5 Bacillus thuringiensis toxins
Abstract
The insecticidal Cry toxins produced by the bacterium Bacillus thuringiensis are comprised of three structural domains. Domain I, a seven-helix bundle, is thought to penetrate the insect epithelial cell plasma membrane through a hairpin composed of alpha-helices 4 and 5, followed by the oligomerization of four hairpin monomers. The alpha-helix 4 has been proposed to line the lumen of the pore, whereas some residues in alpha-helix 5 have been shown to be responsible for oligomerization. Mutation of the Cry1Ac1 alpha-helix 4 amino acid Asn135 to Gln resulted in the loss of toxicity to Manduca sexta, yet binding was still observed. In this study, the equivalent mutation was made in the Cry1Ab5 toxin, and the properties of both wild-type and mutant toxin counterparts were analyzed. Both mutants appeared to bind to M. sexta membrane vesicles, but they were not able to form pores. The ability of both N135Q mutants to oligomerize was also disrupted, providing the first evidence that a residue in alpha-helix 4 can contribute to toxin oligomerization.
Figures

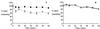
References
-
- Ahmad W, Ellar D J. Directed mutagenesis of selected regions of a Bacillus thuringiensis entomocidal protein. FEMS Microbiol Lett. 1990;68:97–104. - PubMed
-
- Bell R A, Joachim F G. Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Ann Entomol Soc Am. 1976;69:365–373.
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Burton S L, Ellar D J, Li J, Derbyshire D J. N-Acetylgalactosamine on the putative insect receptor aminopeptidase N is recognised by a site on the domain III lectin-like fold of a Bacillus thuringiensis insecticidal toxin. J Mol Biol. 1999;287:1011–1022. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

