N[3-(4'-fluorophenyl)-3-(4'-phenylphenoxy)propyl]sarcosine (NFPS) is a selective persistent inhibitor of glycine transport
- PMID: 11724748
- PMCID: PMC1573075
- DOI: 10.1038/sj.bjp.0704381
N[3-(4'-fluorophenyl)-3-(4'-phenylphenoxy)propyl]sarcosine (NFPS) is a selective persistent inhibitor of glycine transport
Abstract
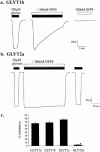
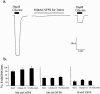
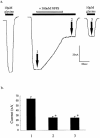
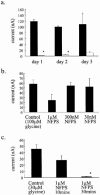
1. The regulation of glycine concentrations within excitatory synapses is poorly understood and it has been proposed that the GLYT1 subtypes of glycine transporters play a critical role in determining resting concentrations of glycine. Selective GLYT1 inhibitors may provide pharmacological tools to probe the dynamics of synaptic glycine concentrations, which may influence the activation properties of NMDA receptor activity. 2. We have characterized the selectivity and mechanism of action of the glycine transport inhibitor N[3-(4'-fluorophenyl)-3-(4'-phenylphenoxy)propyl]sarcosine (NFPS). The glycine transporters, GLYT1a, b and c and GLYT2a were expressed in Xenopus laevis oocytes and two electrode voltage clamp techniques and radiolabelled (3)H-glycine flux measurements were used to characterize the effects of NFPS on glycine transport. 3. NFPS inhibits glycine transport by the GLYT1a, b and c subtypes of glycine transporters, but has no effect on the GLYT2a subtype of transporter. We show that NFPS does not attain its specificity via an interaction with the Na(+), Cl(-) or glycine site, nor does it act at an intracellular site. NFPS inhibition of glycine transport is time and concentration dependent and inhibition of transport by NFPS persists after washout of NFPS from the bath solution, which suggests that inhibition by NFPS is long lasting.
Figures
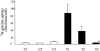
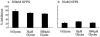
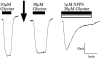
References
-
- AMARA S.G., KUHAR M.J. Neurotransmitter Transporters : Recent Progress. Annu. Rev. Neurosci. 1993;16:73–93. - PubMed
-
- ANDERSON G.P., LINDEN A., RABE K.F. Why are long-acting beta-adrenoceptor agonists long-acting. Eur. Respir. J. 1994;7:569–578. - PubMed
-
- ARAGON M.C., GIMENEZ C., MAYOR F. Stoichiometry of sodium- and chloride-coupled glycine transport in synaptic plasma membrane vesicles derived from rat brain. FEBS Lett. 1987;212:87–90. - PubMed
-
- ATTWELL D., BARBOUR B., SZATKOWSKI M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

