The nonhelical tail domain of keratin 14 promotes filament bundling and enhances the mechanical properties of keratin intermediate filaments in vitro
- PMID: 11724817
- PMCID: PMC2150872
- DOI: 10.1083/jcb.200104063
The nonhelical tail domain of keratin 14 promotes filament bundling and enhances the mechanical properties of keratin intermediate filaments in vitro
Abstract
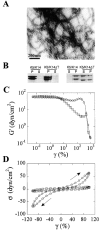
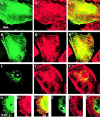
Keratin filaments arise from the copolymerization of type I and II sequences, and form a pancytoplasmic network that provides vital mechanical support to epithelial cells. Keratins 5 and 14 are expressed as a pair in basal cells of stratified epithelia, where they occur as bundled arrays of filaments. In vitro, bundles of K5-K14 filaments can be induced in the absence of cross-linkers, and exhibit enhanced resistance to mechanical strain. This property is not exhibited by copolymers of K5 and tailless K14, in which the nonhelical tail domain has been removed, or copolymers of K5 and K19, a type I keratin featuring a short tail domain. The purified K14 tail domain binds keratin filaments in vitro with specificity (kD approximately 2 microM). When transiently expressed in cultured cells, the K14 tail domain associates with endogenous keratin filaments. Utilization of the K14 tail domain as a bait in a yeast two-hybrid screen pulls out type I keratin sequences from a skin cDNA library. These data suggest that the tail domain of K14 contributes to the ability of K5-K14 filaments to self-organize into large bundles showing enhanced mechanical resilience in vitro.
Figures

Similar articles
-
Pairwise assembly determines the intrinsic potential for self-organization and mechanical properties of keratin filaments.Mol Biol Cell. 2002 Jan;13(1):382-91. doi: 10.1091/mbc.01-10-0522. Mol Biol Cell. 2002. PMID: 11809846 Free PMC article.
-
Defining the properties of the nonhelical tail domain in type II keratin 5: insight from a bullous disease-causing mutation.Mol Biol Cell. 2005 Mar;16(3):1427-38. doi: 10.1091/mbc.e04-06-0498. Epub 2005 Jan 12. Mol Biol Cell. 2005. PMID: 15647384 Free PMC article.
-
The roles of K5 and K14 head, tail, and R/K L L E G E domains in keratin filament assembly in vitro.J Cell Biol. 1992 Oct;119(2):401-14. doi: 10.1083/jcb.119.2.401. J Cell Biol. 1992. PMID: 1383231 Free PMC article.
-
Keratins in health and disease.Curr Opin Cell Biol. 2015 Feb;32:73-81. doi: 10.1016/j.ceb.2014.12.008. Epub 2015 Jan 17. Curr Opin Cell Biol. 2015. PMID: 25599598 Review.
-
Keratins in health and cancer: more than mere epithelial cell markers.Oncogene. 2011 Jan 13;30(2):127-38. doi: 10.1038/onc.2010.456. Epub 2010 Oct 4. Oncogene. 2011. PMID: 20890307 Free PMC article. Review.
Cited by
-
NAT10-mediated mRNA N4-acetylcytidine modification promotes bladder cancer progression.Clin Transl Med. 2022 May;12(5):e738. doi: 10.1002/ctm2.738. Clin Transl Med. 2022. PMID: 35522942 Free PMC article.
-
The mechanical properties of hydrated intermediate filaments: insights from hagfish slime threads.Biophys J. 2003 Sep;85(3):2015-27. doi: 10.1016/S0006-3495(03)74629-3. Biophys J. 2003. PMID: 12944314 Free PMC article.
-
Modes of genetic adaptations underlying functional innovations in the rumen.Sci China Life Sci. 2021 Jan;64(1):1-21. doi: 10.1007/s11427-020-1828-8. Epub 2020 Nov 5. Sci China Life Sci. 2021. PMID: 33165812
-
Truncation of alphaB-crystallin by the myopathy-causing Q151X mutation significantly destabilizes the protein leading to aggregate formation in transfected cells.J Biol Chem. 2008 Apr 18;283(16):10500-12. doi: 10.1074/jbc.M706453200. Epub 2008 Jan 29. J Biol Chem. 2008. PMID: 18230612 Free PMC article.
-
Evolution of the vertebrate beaded filament protein, Bfsp2; comparing the in vitro assembly properties of a "tailed" zebrafish Bfsp2 to its "tailless" human orthologue.Exp Eye Res. 2012 Jan;94(1):192-202. doi: 10.1016/j.exer.2011.12.001. Epub 2011 Dec 11. Exp Eye Res. 2012. PMID: 22182672 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

